Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.saa.2020.118204 Daniel Diaz , David W. Hahn
|
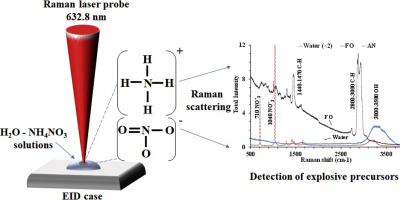
Raman spectroscopy was evaluated as a sensor for detection of ammonium nitrate (NH4NO3, AN), fuel oil (FO), AN-water solutions, and AN- and FO-soil mixtures deposited on materials such as glass, synthetic fabric, cardboard and electrical tape to simulate field conditions of explosives detection. AN is an inorganic oxidizing salt that is commonly used in fertilizers and mining explosives, however, due to its widespread accessibility, AN-based explosives are also utilized for the manufacture of improvised explosive devices (IED). Pure AN crystals were ground to powder size and deposited on several substrates for Raman analysis, whereas FO was analysed in a quartz cuvette. To simulate field conditions samples of powdered AN, AN-water solutions (0.1% to 10.0% AN w/w), AN-soil (50% to 90% AN w/w) and FO-soil (50% to 75% FO w/w) were prepared and deposited on the clutter materials. Raman spectra were acquired at integration times between 0.1 and 30 s, and 3 replicate Raman measurements were carried out for each sample. The spectral window observed ranged from 300 to 3800 cm−1. Several characteristic Raman bands were found, namely, at 710 cm−1 (NO3−) and 1040 cm−1 (NO3−) for AN; 1440–1470 cm−1 (CH) and 2800–3000 cm−1 (CH) for FO; 3000–3500 cm−1 (OH) for water; and 615 cm−1 (CCl), 1254 cm−1 (CH), 1400 cm−1 (CH2) and 1600 cm−1 (aromatic ring) for polyvinyl chloride (PVC, electrical tape). The effect of the AN concentration and integration time on the total and net Raman intensities, relative standard deviation, signal-to-noise ratio and relative limit of detection was evaluated. The relative limit of detection of AN in water was 0.1% (1 mg/g), and absolute limit of detection was 1.0 μg. The optimum integration time (~10 s) for the Raman sensor to capture the analyte signals was estimated based on the Raman figures of merit as a function of the integration time.
中文翻译:

拉曼光谱法检测硝酸铵作为简易爆炸装置中使用的爆炸前体
拉曼光谱法被评估为用于检测硝酸铵(NH 4 NO 3,AN),燃料油(FO),AN水溶液以及沉积在玻璃,合成纤维,纸板和胶带等材料上的AN和FO油混合物,以模拟爆炸物检测的现场条件。AN是一种无机氧化性盐,通常用于肥料和采矿炸药中,但是由于其广泛的可及性,基于AN的炸药也被用于制造简易爆炸装置(IED)。将纯AN晶体研磨成粉末大小,并沉积在几个衬底上以进行拉曼分析,而在石英比色皿中分析FO。为了模拟田间条件下的粉末状AN,AN水溶液(0.1%至10.0%AN w / w),AN土壤(50%至90%AN w / w)和FO土壤(50%至75%FO)的样品制备)并沉积在杂物上。拉曼光谱是在0.1到30 s之间的积分时间获得的,并且对每个样品进行了3次重复拉曼测量。观察到的光谱窗口范围为300至3800 cm-1。几个特征拉曼带被发现,即,在710厘米-1(NO 3 - )和1040厘米-1(NO 3 - )为AN; FO为1440-1470 cm -1(CH)和2800-3000 cm -1(CH); 用水3000–3500 cm -1(OH);和615 cm -1(CCl),1254 cm -1(CH),1400 cm -1(CH 2)和1600 cm -1(芳香环)用于聚氯乙烯(PVC,电工胶带)。评估了AN浓度和积分时间对总和净拉曼强度,相对标准偏差,信噪比和相对检测极限的影响。水中AN的相对检出限为0.1%(1 mg / g),绝对检出限为1.0μg。根据拉曼品质因数作为积分时间的函数,估算拉曼传感器捕获分析物信号的最佳积分时间(约10 s)。































 京公网安备 11010802027423号
京公网安备 11010802027423号