当前位置:
X-MOL 学术
›
JAMA Dermatol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficacy and Safety of Lebrikizumab, a High-Affinity Interleukin 13 Inhibitor, in Adults With Moderate to Severe Atopic Dermatitis: A Phase 2b Randomized Clinical Trial.
JAMA Dermatology ( IF 11.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamadermatol.2020.0079 Emma Guttman-Yassky 1 , Andrew Blauvelt 2 , Lawrence F Eichenfield 3, 4, 5 , Amy S Paller 6 , April W Armstrong 7 , Janice Drew 8 , Ramanan Gopalan 8 , Eric L Simpson 9
JAMA Dermatology ( IF 11.5 ) Pub Date : 2020-04-01 , DOI: 10.1001/jamadermatol.2020.0079 Emma Guttman-Yassky 1 , Andrew Blauvelt 2 , Lawrence F Eichenfield 3, 4, 5 , Amy S Paller 6 , April W Armstrong 7 , Janice Drew 8 , Ramanan Gopalan 8 , Eric L Simpson 9
Affiliation
|
Importance
Interleukin 13 (IL-13) is a central pathogenic mediator driving multiple features of atopic dermatitis (AD) pathophysiology.
Objective
To evaluate the efficacy and safety of lebrikizumab, a novel, high-affinity, monoclonal antibody targeting IL-13 that selectively prevents formation of the IL-13Rα1/IL-4Rα heterodimer receptor signaling complex, in adults with moderate to severe AD.
Design, Setting, and Participants
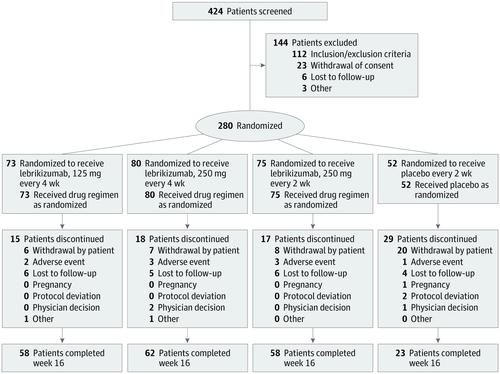
A phase 2b, double-blind, placebo-controlled, dose-ranging randomized clinical trial of lebrikizumab injections every 4 weeks or every 2 weeks was conducted from January 23, 2018, to May 23, 2019, at 57 US centers. Participants were adults 18 years or older with moderate to severe AD.
Interventions
Patients were randomized 2:3:3:3 to placebo every 2 weeks or to subcutaneous injections of lebrikizumab at the following doses: 125 mg every 4 weeks (250-mg loading dose [LD]), 250 mg every 4 weeks (500-mg LD), or 250 mg every 2 weeks (500-mg LD at baseline and week 2).
Main Outcomes and Measures
The primary end point was percentage change in the Eczema Area and Severity Index (EASI) (baseline to week 16). Secondary end points for week 16 included proportion of patients achieving Investigator's Global Assessment score of 0 or 1 (IGA 0/1); EASI improvement of at least 50%, 75%, or 90% from baseline; percentage change in the pruritus numeric rating scale (NRS) score; and pruritus NRS score improvement of at least 4 points. Safety assessments included treatment-emergent adverse events.
Results
A total of 280 patients (mean [SD] age, 39.3 [17.5] years; 166 [59.3%] female) were randomized to placebo (n = 52) or to lebrikizumab at doses of 125 mg every 4 weeks (n = 73), 250 mg every 4 weeks (n = 80), or 250 mg every 2 weeks (n = 75). Compared with placebo (EASI least squares mean [SD] percentage change, -41.1% [56.5%]), lebrikizumab groups showed dose-dependent, statistically significant improvement in the primary end point vs placebo at week 16: 125 mg every 4 weeks (-62.3% [37.3%], P = .02), 250 mg every 4 weeks (-69.2% [38.3%], P = .002), and 250 mg every 2 weeks (-72.1% [37.2%], P < .001). Differences vs placebo-treated patients (2 of 44 [4.5%]) in pruritus NRS improvement of at least 4 points were seen as early as day 2 in the high-dose lebrikizumab group (9 of 59 [15.3%]). Treatment-emergent adverse events were reported in 24 of 52 placebo patients (46.2%) and in lebrikizumab patients as follows: 42 of 73 (57.5%) for 125 mg every 4 weeks, 39 of 80 (48.8%) for 250 mg every 4 weeks, and 46 of 75 (61.3%) for 250 mg every 2 weeks; most were mild to moderate and did not lead to discontinuation. Low rates of injection-site reactions (1 of 52 [1.9%] in the placebo group vs 13 of 228 [5.7%] in all lebrikizumab groups), herpesvirus infections (2 [3.8%] vs 8 [3.5%]), and conjunctivitis (0% vs 6 [2.6%]) were reported.
Conclusions and Relevance
During 16 weeks of treatment, lebrikizumab provided rapid, dose-dependent efficacy across a broad range of clinical manifestations in adult patients with moderate to severe AD and demonstrated a favorable safety profile. These data support the central role of IL-13 in AD pathophysiology. If these findings replicate in phase 3 studies, lebrikizumab may meaningfully advance the standard of care for moderate to severe AD.
Trial Registration
ClinicalTrials.gov Identifier: NCT03443024.
中文翻译:

Lebrikizumab(一种高亲和力的白介素13抑制剂)在中度至重度特应性皮炎成人中的疗效和安全性:2b期随机临床试验。
重要性白介素13(IL-13)是驱动特应性皮炎(AD)病理生理的多种特征的主要致病介质。目的评估lebrikizumab(一种针对IL-13的新型,高亲和力的单克隆抗体)的功效和安全性,该抗体可选择性预防中度至重度AD成年人中IL-13Rα1/IL-4Rα异二聚体受体信号复合物的形成。设计,背景和参与者从2018年1月23日至2019年5月23日,每4周或每2周进行2b期,双盲,安慰剂对照,剂量范围随机的lebrikizumab注射剂临床试验。美国中心。参加者为18岁或以上的中度至重度AD成年人。干预措施患者每2周2:3:3:3被随机分配至安慰剂组或以下列剂量皮下注射来比珠单抗:每4周125 mg(250 mg负荷剂量[LD]),每4周250 mg(500 mg LD)或每2周250 mg(基线和第2周为500 mg LD)。主要结果和措施主要终点是湿疹面积和严重程度指数(EASI)的百分比变化(基线至第16周)。第16周的次要终点包括达到调查者的总体评估评分为0或1(IGA 0/1)的患者比例;EASI与基线相比至少提高了50%,75%或90%;瘙痒症数字评分量表(NRS)分数的变化百分比;瘙痒症的NRS评分至少提高4分。安全性评估包括治疗紧急不良事件。结果共有280例患者(平均[SD]年龄,39.3 [17.5]岁; 166 [59.3%]女性)随机接受安慰剂(n = 52)或每4周服用125 mg来布列单抗(n = 73) ),每4周250毫克(n = 80),或每2周250 mg(n = 75)。与安慰剂比较(EASI最小二乘均值[SD]百分比变化,-41.1%[56.5%]),来普列珠单抗组在第16周时与安慰剂相比在主要终点方面显示出剂量依赖性,统计学上的显着改善:每4周125 mg( -62.3%[37.3%],P = .02),每4周250 mg(-69.2%[38.3%],P = .002)和每2周250 mg(-72.1%[37.2%],P) <.001)。在大剂量的lebrikizumab组中,早在第2天就发现与安慰剂治疗的患者(44例中的2例,占44 [4.5%])的NRS改善至少4分之间的差异(59例中的9例,占[15.3%])。据报道,在52名安慰剂患者中有24名(46.2%)和来瑞布珠单抗患者中出现了治疗突发性不良事件:每4周125 mg服用73剂中有42剂(57.5%),每4周服用250 mg服用80剂中的39剂(48.8%)周和75中的46(61。3%),每2周250毫克;多数为轻度至中度,未导致停药。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。多数为轻度至中度,未导致停药。注射部位反应的发生率较低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。多数为轻度至中度,未导致停药。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。注射部位反应的发生率较低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。据报道,疱疹病毒感染(2 [3.8%]比8 [3.5%])和结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。据报道,疱疹病毒感染(2 [3.8%]比8 [3.5%])和结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。在中度至重度AD成人患者的广泛临床表现中,剂量依赖性疗效均得到证实,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。在中度至重度AD成人患者的广泛临床表现中,剂量依赖性疗效均得到证实,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来波珠单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。
更新日期:2020-04-01
中文翻译:

Lebrikizumab(一种高亲和力的白介素13抑制剂)在中度至重度特应性皮炎成人中的疗效和安全性:2b期随机临床试验。
重要性白介素13(IL-13)是驱动特应性皮炎(AD)病理生理的多种特征的主要致病介质。目的评估lebrikizumab(一种针对IL-13的新型,高亲和力的单克隆抗体)的功效和安全性,该抗体可选择性预防中度至重度AD成年人中IL-13Rα1/IL-4Rα异二聚体受体信号复合物的形成。设计,背景和参与者从2018年1月23日至2019年5月23日,每4周或每2周进行2b期,双盲,安慰剂对照,剂量范围随机的lebrikizumab注射剂临床试验。美国中心。参加者为18岁或以上的中度至重度AD成年人。干预措施患者每2周2:3:3:3被随机分配至安慰剂组或以下列剂量皮下注射来比珠单抗:每4周125 mg(250 mg负荷剂量[LD]),每4周250 mg(500 mg LD)或每2周250 mg(基线和第2周为500 mg LD)。主要结果和措施主要终点是湿疹面积和严重程度指数(EASI)的百分比变化(基线至第16周)。第16周的次要终点包括达到调查者的总体评估评分为0或1(IGA 0/1)的患者比例;EASI与基线相比至少提高了50%,75%或90%;瘙痒症数字评分量表(NRS)分数的变化百分比;瘙痒症的NRS评分至少提高4分。安全性评估包括治疗紧急不良事件。结果共有280例患者(平均[SD]年龄,39.3 [17.5]岁; 166 [59.3%]女性)随机接受安慰剂(n = 52)或每4周服用125 mg来布列单抗(n = 73) ),每4周250毫克(n = 80),或每2周250 mg(n = 75)。与安慰剂比较(EASI最小二乘均值[SD]百分比变化,-41.1%[56.5%]),来普列珠单抗组在第16周时与安慰剂相比在主要终点方面显示出剂量依赖性,统计学上的显着改善:每4周125 mg( -62.3%[37.3%],P = .02),每4周250 mg(-69.2%[38.3%],P = .002)和每2周250 mg(-72.1%[37.2%],P) <.001)。在大剂量的lebrikizumab组中,早在第2天就发现与安慰剂治疗的患者(44例中的2例,占44 [4.5%])的NRS改善至少4分之间的差异(59例中的9例,占[15.3%])。据报道,在52名安慰剂患者中有24名(46.2%)和来瑞布珠单抗患者中出现了治疗突发性不良事件:每4周125 mg服用73剂中有42剂(57.5%),每4周服用250 mg服用80剂中的39剂(48.8%)周和75中的46(61。3%),每2周250毫克;多数为轻度至中度,未导致停药。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。多数为轻度至中度,未导致停药。注射部位反应的发生率较低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。多数为轻度至中度,未导致停药。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。注射部位反应的发生率低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。注射部位反应的发生率较低(安慰剂组为52个中的1个[1.9%],所有勒布雷珠单抗组均为228个中的13个[5.7%]),疱疹病毒感染(2个[3.8%]和8个[3.5%]),以及据报道结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。据报道,疱疹病毒感染(2 [3.8%]比8 [3.5%])和结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。据报道,疱疹病毒感染(2 [3.8%]比8 [3.5%])和结膜炎(0%比6 [2.6%])。结论与相关性在治疗的16周中,来布列单抗在中度至重度AD成人患者的广泛临床表现中提供了快速,剂量依赖性的疗效,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。在中度至重度AD成人患者的广泛临床表现中,剂量依赖性疗效均得到证实,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来布列单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。在中度至重度AD成人患者的广泛临床表现中,剂量依赖性疗效均得到证实,并显示出良好的安全性。这些数据支持IL-13在AD病理生理中的核心作用。如果这些发现在第3期研究中得以重复,那么来波珠单抗可能会有意义地提高中度至重度AD的护理标准。试用注册ClinicalTrials.gov标识符:NCT03443024。











































 京公网安备 11010802027423号
京公网安备 11010802027423号