当前位置:
X-MOL 学术
›
Process Saf. Environ. Prot.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen Scavenging Performance of Carboxylic Acids Formed During the Degradation of Monoethylene Glycol Regeneration Products
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.psep.2020.02.022 Ammar Al Helal , Peter Milner , Ahmed Barifcani
Process Safety and Environmental Protection ( IF 6.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.psep.2020.02.022 Ammar Al Helal , Peter Milner , Ahmed Barifcani
|
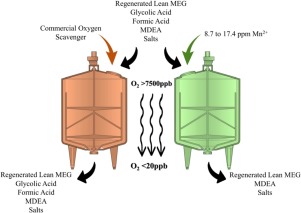
Abstract Previous studies have demonstrated that formic and glycolic acids could scavenge oxygen from aqueous solutions via complex radical mechanisms similar to that of erythorbic acid. Therefore, it is important to study the feasibility of using these common thermal degradation products obtained from the regeneration of monoethylene glycol (MEG). By replacing commercially used oxygen scavengers, such as sulfites, with these organic acids, the toxicity and subsequent environmental impact of oil and gas products could be easily reduced. Furthermore, these organic acids are rapid oxygen scavengers at low concentration, and they could be cost-effective; in addition, the chemical inventory could be reduced and project costs could be further optimized. The experimental results confirmed the potential of formic and glycolic acids to be used as oxygen scavengers downstream of the MEG regeneration process in the presence of manganese (II) ions as catalyst. Of the two commercial aqueous industrial oxygen scavengers tested, diethylhydroxylamine was an extremely rapid oxygen scavenger in salty aged lean MEG solutions under alkaline conditions, whereas methyl ethyl ketoxime exhibited extremely poor performance under similar conditions; therefore, it was ruled out as potential oxygen scavenger.
中文翻译:

单乙二醇再生产物降解过程中生成的羧酸的除氧性能
摘要 先前的研究表明,甲酸和乙醇酸可以通过类似于异抗坏血酸的复杂自由基机制从水溶液中清除氧气。因此,重要的是研究使用这些从单乙二醇 (MEG) 再生中获得的常见热降解产物的可行性。通过用这些有机酸代替商业上使用的除氧剂,例如亚硫酸盐,可以轻松降低石油和天然气产品的毒性和随后对环境的影响。此外,这些有机酸在低浓度下是快速除氧剂,并且具有成本效益;此外,可以减少化学品库存并进一步优化项目成本。实验结果证实了甲酸和乙醇酸在锰 (II) 离子作为催化剂存在下用作 MEG 再生过程下游的除氧剂的潜力。在测试的两种商业水性工业除氧剂中,二乙基羟胺在碱性条件下在含盐老化的贫 MEG 溶液中是一种极快的除氧剂,而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。
更新日期:2020-05-01
中文翻译:

单乙二醇再生产物降解过程中生成的羧酸的除氧性能
摘要 先前的研究表明,甲酸和乙醇酸可以通过类似于异抗坏血酸的复杂自由基机制从水溶液中清除氧气。因此,重要的是研究使用这些从单乙二醇 (MEG) 再生中获得的常见热降解产物的可行性。通过用这些有机酸代替商业上使用的除氧剂,例如亚硫酸盐,可以轻松降低石油和天然气产品的毒性和随后对环境的影响。此外,这些有机酸在低浓度下是快速除氧剂,并且具有成本效益;此外,可以减少化学品库存并进一步优化项目成本。实验结果证实了甲酸和乙醇酸在锰 (II) 离子作为催化剂存在下用作 MEG 再生过程下游的除氧剂的潜力。在测试的两种商业水性工业除氧剂中,二乙基羟胺在碱性条件下在含盐老化的贫 MEG 溶液中是一种极快的除氧剂,而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。而甲基乙基酮肟在类似条件下表现出极差的性能;因此,它被排除为潜在的氧气清除剂。





























 京公网安备 11010802027423号
京公网安备 11010802027423号