当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-25 , DOI: 10.1038/s41467-020-14848-2 Shi Fang 1 , Xiaorong Zhu 2 , Xiaokang Liu 1 , Jian Gu 3 , Wei Liu 1 , Danhao Wang 1 , Wei Zhang 1 , Yue Lin 3 , Junling Lu 3 , Shiqiang Wei 1 , Yafei Li 2 , Tao Yao 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-25 , DOI: 10.1038/s41467-020-14848-2 Shi Fang 1 , Xiaorong Zhu 2 , Xiaokang Liu 1 , Jian Gu 3 , Wei Liu 1 , Danhao Wang 1 , Wei Zhang 1 , Yue Lin 3 , Junling Lu 3 , Shiqiang Wei 1 , Yafei Li 2 , Tao Yao 1
Affiliation
|
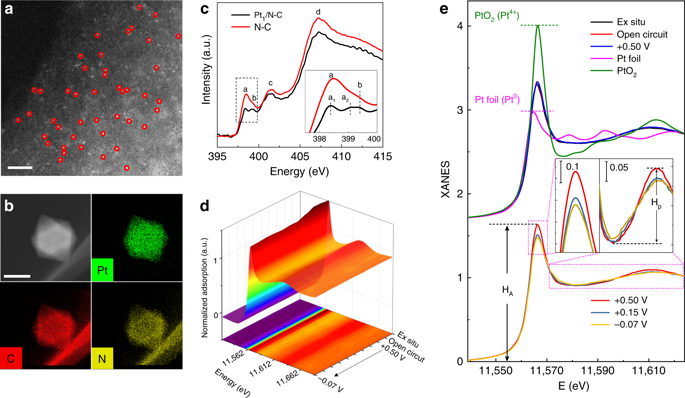
Single-atom catalysts offering intriguing activity and selectivity are subject of intense investigation. Understanding the nature of single-atom active site and its dynamics under working state are crucial to improving their catalytic performances. Here, we identify at atomic level a general evolution of single atom into a near-free state under electrocatalytic hydrogen evolution condition, via operando synchrotron X-ray absorption spectroscopy. We uncover that the single Pt atom tends to dynamically release from the nitrogen-carbon substrate, with the geometric structure less coordinated to support and electronic property closer to zero valence, during the reaction. Theoretical simulations support that the Pt sites with weakened Pt-support interaction and more 5d density are the real active centers. The single-atom Pt catalyst exhibits very high hydrogen evolution activity with only 19 mV overpotential in 0.5 M H2SO4 and 46 mV in 1.0 M NaOH at 10 mA cm-2, and long-term durability in wide-pH electrolytes.
中文翻译:

在电化学氢释放反应过程中发现近乎自由的铂单原子动力学。
提供令人感兴趣的活性和选择性的单原子催化剂是深入研究的主题。了解单原子活性位点的性质及其在工作状态下的动力学对于提高其催化性能至关重要。在这里,我们通过操作同步加速器X射线吸收光谱在电催化氢放出条件下,在原子水平上确定了单个原子向近自由状态的一般演化。我们发现,在反应过程中,单个Pt原子趋于从氮-碳底物动态释放,其几何结构不太协调地支撑,电子特性更接近零价。理论模拟表明,Pt-支撑相互作用减弱且5d密度更高的Pt部位是真正的活性中心。
更新日期:2020-02-25
中文翻译:

在电化学氢释放反应过程中发现近乎自由的铂单原子动力学。
提供令人感兴趣的活性和选择性的单原子催化剂是深入研究的主题。了解单原子活性位点的性质及其在工作状态下的动力学对于提高其催化性能至关重要。在这里,我们通过操作同步加速器X射线吸收光谱在电催化氢放出条件下,在原子水平上确定了单个原子向近自由状态的一般演化。我们发现,在反应过程中,单个Pt原子趋于从氮-碳底物动态释放,其几何结构不太协调地支撑,电子特性更接近零价。理论模拟表明,Pt-支撑相互作用减弱且5d密度更高的Pt部位是真正的活性中心。

































 京公网安备 11010802027423号
京公网安备 11010802027423号