Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.cej.2020.124568
Yunxiang Nie , Shuai Li , Jinfeng Dai , Dedong He , Yi Mei
|
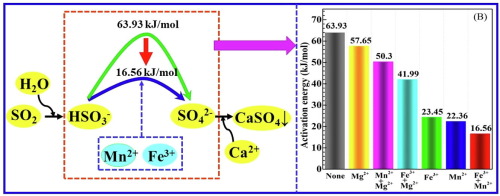
Wet phosphate rock flue gas desulfurization is a new and promising technology, which has drawn increasing attention in recent years. The effect of Mn2+, Fe3+ and Mg2+ dissolved from phosphate rock on removal efficiency of SO2 were systematically investigated in this paper. The XRD, FT-IR, SEM, XPS, IC and ICP were used to characterize the reacted products, demonstrating that these metal ions have promotive effect on the purification of SO2. Especially, trace Mn2+ and Fe3+ ions can rose up about 19% – 31.5% SO2 absorption capacity compared to the system without metal ions. Furthermore, the catalytic effect of these metal ions on the oxidation of S(IV) were compared, by adding them to the sodium bisulfite aqueous solution system. The order of catalytic capability of metal ions to S(IV) is: Mn2+ > Fe3+ > Mg2+. Interestingly, in the simultaneous presence of Mn2+ and Fe3+, the catalytic synergism between them toward S(IV) oxidation is exhibited. And only 16.56 kJ/mol of activation energy is needed, which is reduced by 74.1% compared to systems without catalyst. Consequently, a satisfactory result for absorption of SO2 can be obtained although the concentrations of Mn2+ and Fe3+ in phosphate rock slurry is low. Additionally, the catalytic mechanism of Mn2+ and Fe3+ towards S(IV) was investigated by reaction barrier and free energy calculation.
中文翻译:

Mn 2 +,Fe 3+和Mg 2+离子对以磷矿浆为吸收剂脱硫的催化作用
湿法磷酸盐岩烟气脱硫是一项新的有前途的技术,近年来引起了越来越多的关注。本文系统地研究了磷矿中溶解的Mn 2 +,Fe 3+和Mg 2+对SO 2去除效率的影响。用XRD,FT-IR,SEM,XPS,IC和ICP对反应产物进行表征,表明这些金属离子对SO 2的纯化具有促进作用。尤其是痕量的Mn 2+和Fe 3+离子可以上升约19%– 31.5%的SO 2。与没有金属离子的系统相比,其吸收能力强。此外,通过将它们添加到亚硫酸氢钠水溶液体系中,比较了这些金属离子对S(IV)氧化的催化作用。金属离子对S(IV)的催化能力顺序为:Mn 2+ > Fe 3+ > Mg 2+。有趣的是,在同时存在Mn 2+和Fe 3+的情况下,它们之间表现出对S(IV)氧化的催化协同作用。并且仅需要16.56 kJ / mol的活化能,与没有催化剂的系统相比,降低了74.1%。因此,尽管Mn 2+的浓度可以得到令人满意的SO 2吸收结果。磷矿浆中的Fe 3+含量较低。此外,通过反应势垒和自由能计算研究了Mn 2+和Fe 3+对S(IV)的催化机理。

































 京公网安备 11010802027423号
京公网安备 11010802027423号