当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functioning Mechanism of the Secondary Aqueous Zn - β-MnO2 Battery.
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-02-24 , DOI: 10.1021/acsami.9b22758 Longyan Li 1 , Tuan K A Hoang 2 , Jian Zhi 2 , Mei Han 2 , Shengkai Li 2 , P Chen 2
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2020-02-24 , DOI: 10.1021/acsami.9b22758 Longyan Li 1 , Tuan K A Hoang 2 , Jian Zhi 2 , Mei Han 2 , Shengkai Li 2 , P Chen 2
Affiliation
|
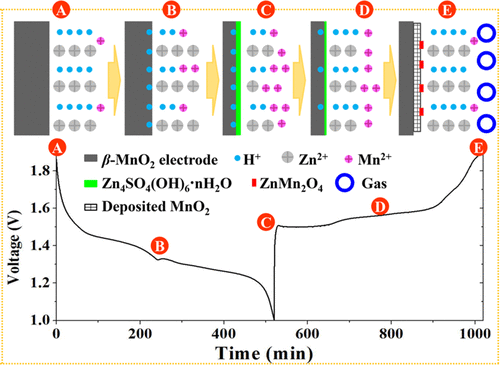
Rechargeable aqueous Zn-MnO2 batteries are a promising candidate for large-scale energy storage systems thanks to their unparalleled features such as high safety, low cost, and environmental friendliness. Considering the controversies surrounding the mechanism of this battery containing a mildly acidic electrolyte, the electrochemical behavior of this type of battery using β-MnO2 as the cathode is systematically investigated. The results indicate that the reversible intercalation of Zn2+ ions into MnO2 is not likely to take place in the aqueous system. We conclude that it is the existence of water molecule and its participation in the electrochemical reactions, for instance, the reversible insertion of proton into MnO2 and the electrolysis of water that makes the mechanism of aqueous Zn-MnO2 batteries complicated. Besides, the capacity fading of this mildly acidic Zn-MnO2 battery is assigned to the generation of the inert layer of Zn4SO4(OH)6nH2O and the ZnMn2O4 on the cathode via electrochemical conversion reactions, the dissolution of the active material during discharging, and the release of gases. When Mn2+ ions are available in the electrolyte, they will be electrodeposited on the cathode during charging process and the kinetics of the electrochemical reactions of the electrode are improved, leading to the higher electrochemical performance of the battery.
中文翻译:

Zn-β-MnO2二次电池的功能机理。
Zn-MnO2水性可充电电池由于其无与伦比的特性(如高安全性,低成本和环境友好性)而成为大规模储能系统的有希望的候选者。考虑到围绕这种包含弱酸性电解质的电池机理的争议,系统地研究了使用β-MnO2作为阴极的这类电池的电化学行为。结果表明,在水性体系中不可能将Zn2 +离子可逆地嵌入MnO2中。我们得出的结论是,水分子的存在及其在电化学反应中的参与,例如质子向MnO2的可逆插入以及水的电解使Zn-MnO2水溶液电池的机理变得复杂。除了,这种弱酸性Zn-MnO2电池的容量衰减归因于通过电化学转化反应在阴极上生成Zn4SO4(OH)6nH2O和ZnMn2O4惰性层,放电过程中活性物质的溶解以及释放气体。当电解质中存在Mn2 +离子时,它们将在充电过程中电沉积在阴极上,从而改善了电极的电化学反应动力学,从而提高了电池的电化学性能。
更新日期:2020-03-05
中文翻译:

Zn-β-MnO2二次电池的功能机理。
Zn-MnO2水性可充电电池由于其无与伦比的特性(如高安全性,低成本和环境友好性)而成为大规模储能系统的有希望的候选者。考虑到围绕这种包含弱酸性电解质的电池机理的争议,系统地研究了使用β-MnO2作为阴极的这类电池的电化学行为。结果表明,在水性体系中不可能将Zn2 +离子可逆地嵌入MnO2中。我们得出的结论是,水分子的存在及其在电化学反应中的参与,例如质子向MnO2的可逆插入以及水的电解使Zn-MnO2水溶液电池的机理变得复杂。除了,这种弱酸性Zn-MnO2电池的容量衰减归因于通过电化学转化反应在阴极上生成Zn4SO4(OH)6nH2O和ZnMn2O4惰性层,放电过程中活性物质的溶解以及释放气体。当电解质中存在Mn2 +离子时,它们将在充电过程中电沉积在阴极上,从而改善了电极的电化学反应动力学,从而提高了电池的电化学性能。















































 京公网安备 11010802027423号
京公网安备 11010802027423号