当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Urolithin A-induced mitophagy suppresses apoptosis and attenuates intervertebral disc degeneration via the AMPK signaling pathway.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.freeradbiomed.2020.02.024 Jialiang Lin 1 , Jinru Zhuge 2 , Xuanqi Zheng 1 , Yuhao Wu 3 , Zengjie Zhang 1 , Tianzhen Xu 1 , Zaher Meftah 1 , Hongming Xu 4 , Yaosen Wu 1 , Naifeng Tian 1 , Weiyang Gao 1 , Yifei Zhou 1 , Xiaolei Zhang 5 , Xiangyang Wang 1
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-02-24 , DOI: 10.1016/j.freeradbiomed.2020.02.024 Jialiang Lin 1 , Jinru Zhuge 2 , Xuanqi Zheng 1 , Yuhao Wu 3 , Zengjie Zhang 1 , Tianzhen Xu 1 , Zaher Meftah 1 , Hongming Xu 4 , Yaosen Wu 1 , Naifeng Tian 1 , Weiyang Gao 1 , Yifei Zhou 1 , Xiaolei Zhang 5 , Xiangyang Wang 1
Affiliation
|
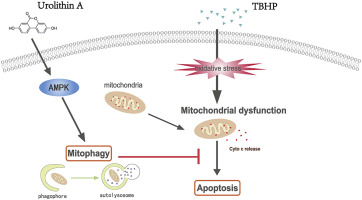
Intervertebral disc degeneration (IDD) is a major cause of low back pain (LBP), and effective therapies are still lacking. Previous studies reported that mitochondrial dysfunction contributes to apoptosis, and urolithin A (UA) specifically induces mitophagy. Herein, we aimed to investigate the protective effect of UA-induced mitophagy on tert-butyl hydroperoxide (TBHP)-induced apoptosis in nucleus pulposus (NP) cells in vitro and a rat model of IDD in vivo. Mitochondrial function, apoptosis, and mitophagy were measured in UA-treated NP cells by western blotting and immunofluorescence; the therapeutic effects of UA on IDD were assessed in rats with puncture-induced IDD. The results showed that UA could activate mitophagy in primary NP cells, and UA treatment inhibited TBHP-induced mitochondrial dysfunction and the intrinsic apoptosis pathway. Mechanistically, we revealed that UA promoted mitophagy by activating AMPK signaling in TBHP-induced NP cells. In vivo, UA was shown to effectively alleviate the progression of puncture-induced IDD in rats. Taken together, our results suggest that UA could be a novel and effective therapeutic strategy for IDD.
中文翻译:

尿石素A诱导的细胞吞噬抑制细胞凋亡,并通过AMPK信号通路减弱椎间盘退变。
椎间盘退变(IDD)是下腰痛(LBP)的主要原因,仍然缺乏有效的治疗方法。先前的研究报道,线粒体功能障碍导致细胞凋亡,而尿石素A(UA)特异性诱导线粒体吞噬。本文中,我们旨在研究UA诱导的线粒体对叔丁基氢过氧化物(TBHP)诱导的髓核(NP)细胞凋亡的体内保护作用以及体内IDD的大鼠模型的保护作用。通过Western印迹和免疫荧光检测UA处理的NP细胞的线粒体功能,凋亡和线粒体。在穿刺诱导的IDD大鼠中评估了UA对IDD的治疗作用。结果表明,UA可以激活原代NP细胞的线粒体吞噬,UA处理可以抑制TBHP诱导的线粒体功能障碍和固有的凋亡途径。从机制上讲,我们揭示了UA通过激活TBHP诱导的NP细胞中的AMPK信号传导来促进线粒体的吞噬。在体内,UA被证明可有效减轻大鼠穿刺诱导IDD的进展。综上所述,我们的结果表明,UA可能是IDD的一种新颖有效的治疗策略。
更新日期:2020-02-24
中文翻译:

尿石素A诱导的细胞吞噬抑制细胞凋亡,并通过AMPK信号通路减弱椎间盘退变。
椎间盘退变(IDD)是下腰痛(LBP)的主要原因,仍然缺乏有效的治疗方法。先前的研究报道,线粒体功能障碍导致细胞凋亡,而尿石素A(UA)特异性诱导线粒体吞噬。本文中,我们旨在研究UA诱导的线粒体对叔丁基氢过氧化物(TBHP)诱导的髓核(NP)细胞凋亡的体内保护作用以及体内IDD的大鼠模型的保护作用。通过Western印迹和免疫荧光检测UA处理的NP细胞的线粒体功能,凋亡和线粒体。在穿刺诱导的IDD大鼠中评估了UA对IDD的治疗作用。结果表明,UA可以激活原代NP细胞的线粒体吞噬,UA处理可以抑制TBHP诱导的线粒体功能障碍和固有的凋亡途径。从机制上讲,我们揭示了UA通过激活TBHP诱导的NP细胞中的AMPK信号传导来促进线粒体的吞噬。在体内,UA被证明可有效减轻大鼠穿刺诱导IDD的进展。综上所述,我们的结果表明,UA可能是IDD的一种新颖有效的治疗策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号