当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Distribution of Spin Density on Phenoxyl Radicals Affects the Selectivity of Aerobic Oxygenation of Phenols.
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.inorgchem.9b02422 Yongtao Wang 1 , Jun Guan 1 , Bingbao Mei 2 , Mengtian Fan 1 , Rui Lu 1 , Renfeng Du 1 , Kaizhou Chen 1 , Jia Yao 1 , Zheng Jiang 2 , Haoran Li 1, 3
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2020-02-21 , DOI: 10.1021/acs.inorgchem.9b02422 Yongtao Wang 1 , Jun Guan 1 , Bingbao Mei 2 , Mengtian Fan 1 , Rui Lu 1 , Renfeng Du 1 , Kaizhou Chen 1 , Jia Yao 1 , Zheng Jiang 2 , Haoran Li 1, 3
Affiliation
|
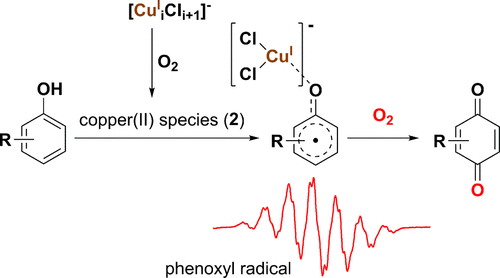
Phenoxyl radical was generally suggested as the intermediate during copper-catalyzed aerobic oxygenation of phenols. However, the substrate-dependent selectivity has not been well interpreted, due to insufficient characterization of the radical intermediate under reaction conditions. When studying the CuCl-LiCl-catalyzed aerobic phenol oxidation, we obtained EPR spectra of phenoxyl radicals generated by oxidizing phenols with the preactivated catalyst. Upon correlation to the selectivity of benzoquinone, the hyperfine coupling constant of para-site proton (aH, para) was found to be better than the Hammett constant. The catalysis mechanism was studied based on EPR detection and the reaction results of phenoxyl radicals under N2 or O2 atmosphere. It appeared that the chemoselectivity depended on the attack of activated dioxygen on phenoxyl radicals, and the activation of dioxygen by [CunCln+1]- (n = 1, 2, 3) was suggested as the rate-determining step. Understanding of the substrate-dependent selectivity contributed to predicting the chemoselectivity in the aerobic oxidation of phenols.
中文翻译:

自旋密度在苯氧基自由基上的分布会影响苯酚的有氧氧化的选择性。
通常认为苯氧基自由基是铜催化的苯酚的有氧氧合的中间体。然而,由于自由基中间体在反应条件下的表征不足,因此没有很好地解释与底物有关的选择性。在研究CuCl-LiCl催化的好氧苯酚氧化时,我们获得了用预活化催化剂氧化酚所产生的苯氧基自由基的EPR谱。与苯醌的选择性相关时,发现对位质子(aH,对)的超精细偶合常数优于Hammett常数。基于EPR检测和N2或O2气氛下苯氧基自由基的反应机理,研究了其催化机理。看来化学选择性取决于活化的双氧对苯氧基自由基的攻击,建议通过[CunCln + 1]-(n = 1,2,3)激活双氧作为速率确定步骤。对底物依赖性选择性的理解有助于预测苯酚的有氧氧化中的化学选择性。
更新日期:2020-02-23
中文翻译:

自旋密度在苯氧基自由基上的分布会影响苯酚的有氧氧化的选择性。
通常认为苯氧基自由基是铜催化的苯酚的有氧氧合的中间体。然而,由于自由基中间体在反应条件下的表征不足,因此没有很好地解释与底物有关的选择性。在研究CuCl-LiCl催化的好氧苯酚氧化时,我们获得了用预活化催化剂氧化酚所产生的苯氧基自由基的EPR谱。与苯醌的选择性相关时,发现对位质子(aH,对)的超精细偶合常数优于Hammett常数。基于EPR检测和N2或O2气氛下苯氧基自由基的反应机理,研究了其催化机理。看来化学选择性取决于活化的双氧对苯氧基自由基的攻击,建议通过[CunCln + 1]-(n = 1,2,3)激活双氧作为速率确定步骤。对底物依赖性选择性的理解有助于预测苯酚的有氧氧化中的化学选择性。















































 京公网安备 11010802027423号
京公网安备 11010802027423号