当前位置:
X-MOL 学术
›
Adv. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Uncovering the Potential of M1-Site-Activated NASICON Cathodes for Zn-Ion Batteries.
Advanced Materials ( IF 27.4 ) Pub Date : 2020-02-20 , DOI: 10.1002/adma.201907526 Pu Hu 1 , Zheyi Zou 2 , Xingwei Sun 3 , Da Wang 2 , Jun Ma 1 , Qingyu Kong 4 , Dongdong Xiao 5 , Lin Gu 5 , Xinhong Zhou 3 , Jingwen Zhao 1 , Shanmu Dong 1 , Bing He 6 , Maxim Avdeev 7, 8 , Siqi Shi 2 , Guanglei Cui 1 , Liquan Chen 9
Advanced Materials ( IF 27.4 ) Pub Date : 2020-02-20 , DOI: 10.1002/adma.201907526 Pu Hu 1 , Zheyi Zou 2 , Xingwei Sun 3 , Da Wang 2 , Jun Ma 1 , Qingyu Kong 4 , Dongdong Xiao 5 , Lin Gu 5 , Xinhong Zhou 3 , Jingwen Zhao 1 , Shanmu Dong 1 , Bing He 6 , Maxim Avdeev 7, 8 , Siqi Shi 2 , Guanglei Cui 1 , Liquan Chen 9
Affiliation
|
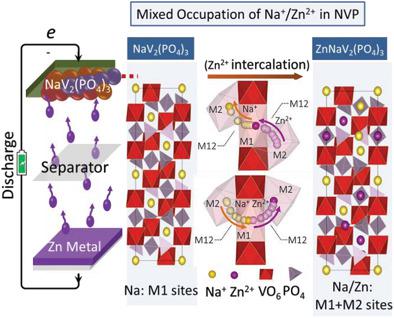
There is a long-standing consciousness that the rhombohedral NASICON-type compounds as promising cathodes for Li+ /Na+ batteries should have inactive M1(6b) sites with ion (de)intercalation occurring only in the M2 (18e) sites. Of particular significance is that M1 sites active for charge/discharge are commonly considered undesirable because the ion diffusion tends to be disrupted by the irregular occupation of channels, which accelerates the deterioration of battery. However, it is found that the structural stability can be substantially improved by the mixed occupation of Na+ /Zn2+ at both M1 and M2 when using NaV2 (PO4 )3 (NVP) as a cathode for Zn-ion batteries. The results of atomic-scale scanning transmission electron microscopy, analysis of ab initio molecular dynamics simulations, and an accurate bond-valence-based structural model reveal that the improvement is due to the facile migration of Zn2+ in NVP, which is enabled by a concerted Na+ /Zn2+ transfer mechanism. In addition, significant improvement of the electronic conductivity and mechanical properties is achieved in Zn2+ -intercalated ZnNaV2 (PO4 )3 in comparison with those of Na3 V2 (PO4 )3 . This work not only provides in-depth insight into Zn2+ intercalation and dynamics in NVP unlocked by activating the M1 sites, but also opens a new route toward design of improved NASICON cathodes.
中文翻译:

揭示M1活化的NASICON阴极用于锌离子电池的潜力。
长期以来,人们一直意识到菱形NASICON型化合物作为有前途的Li + / Na +电池阴极应具有不活动的M1(6b)位点,且仅在M2(18e)位点上发生离子(脱嵌)。特别重要的是,通常认为对M1充电/放电有效的位点是不希望有的,因为离子的扩散往往会被不规则占据的通道所破坏,从而加速了电池的老化。然而,发现当使用NaV 2(PO 4)3(NVP)作为Zn离子电池的阴极时,通过在M1和M2处同时占据Na + / Zn 2+,可以显着改善结构稳定性。原子级扫描透射电子显微镜的结果,从头算分子动力学模拟的分析,准确的基于键价的结构模型表明,这种改善是由于NVP中Zn2 +的迁移容易,这是由协调的Na + / Zn2 +转移机制实现的。另外,与Na 3 V 2(PO 4)3相比,嵌入Zn 2+的ZnNaV 2(PO 4)3具有显着改善的电子导电性和机械性能。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。与Na3 V2(PO4)3相比,嵌入Zn2 +的ZnNaV2(PO4)3的电导率和机械性能有了显着提高。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。与Na3 V2(PO4)3相比,嵌入Zn2 +的ZnNaV2(PO4)3的电导率和机械性能有了显着改善。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。
更新日期:2020-04-08
中文翻译:

揭示M1活化的NASICON阴极用于锌离子电池的潜力。
长期以来,人们一直意识到菱形NASICON型化合物作为有前途的Li + / Na +电池阴极应具有不活动的M1(6b)位点,且仅在M2(18e)位点上发生离子(脱嵌)。特别重要的是,通常认为对M1充电/放电有效的位点是不希望有的,因为离子的扩散往往会被不规则占据的通道所破坏,从而加速了电池的老化。然而,发现当使用NaV 2(PO 4)3(NVP)作为Zn离子电池的阴极时,通过在M1和M2处同时占据Na + / Zn 2+,可以显着改善结构稳定性。原子级扫描透射电子显微镜的结果,从头算分子动力学模拟的分析,准确的基于键价的结构模型表明,这种改善是由于NVP中Zn2 +的迁移容易,这是由协调的Na + / Zn2 +转移机制实现的。另外,与Na 3 V 2(PO 4)3相比,嵌入Zn 2+的ZnNaV 2(PO 4)3具有显着改善的电子导电性和机械性能。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。与Na3 V2(PO4)3相比,嵌入Zn2 +的ZnNaV2(PO4)3的电导率和机械性能有了显着提高。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。与Na3 V2(PO4)3相比,嵌入Zn2 +的ZnNaV2(PO4)3的电导率和机械性能有了显着改善。这项工作不仅提供了对通过激活M1位而解锁的NVP中Zn2 +嵌入和动力学的深入了解,而且为设计改进的NASICON阴极开辟了一条新途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号