当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and anti‐proliferative activity studies of 2‐(2‐(trifluoromethyl)‐6‐(substituted)imidazo[1,2‐b]pyridazin‐3‐yl)‐N‐(substituted)acetamide derivatives
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-21 , DOI: 10.1002/jhet.3920 Dattatraya D. Gaikwad 1 , Umakant D. Pawar 2 , Sadhana L. Chavan 1 , Chandrakant D. Pawar 3 , Dattatraya N. Pansare 1 , Rohini N. Shelke 1 , Santosh L. Chavan 4 , Ashok M. Zine 5
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-21 , DOI: 10.1002/jhet.3920 Dattatraya D. Gaikwad 1 , Umakant D. Pawar 2 , Sadhana L. Chavan 1 , Chandrakant D. Pawar 3 , Dattatraya N. Pansare 1 , Rohini N. Shelke 1 , Santosh L. Chavan 4 , Ashok M. Zine 5
Affiliation
|
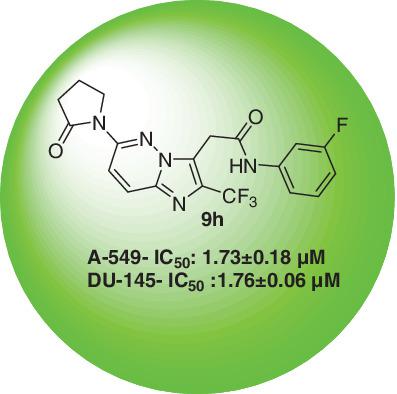
A series of novel imidazo[1,2‐b]pyridazin‐3‐yl acetamide derivatives (9a‐9j) were synthesized from a 3,6‐dichloropyridazine. We have developed a simple strategy for the synthesis of functionally diverse imidazole, and pyridiazine derivatives were reported via a series of steps. The work involves bicyclic imidazo‐pyridazine ring formation, halogenation, cynation, hydrolysis, peptide coupling, and Buchwald reaction. The structure of the synthesized compounds was confirmed by IR, 1H NMR, 13C NMR,19F NMR, mass spectra, and elemental analysis, and purity is checked by HPLC. All synthesized compounds were screened for anticancer activity against A‐549 and Du‐145 cancer cell lines by MTT assay. The preliminary bioassay suggests that most of the compounds show anti‐proliferation with different degrees; doxorubicin was used as positive control. The synthesized compound shows IC50 values in the range of 1.74μM to 16.17μM in both cell lines. The compounds 9e, 9g, and 9h were active compared with doxorubicin in both the cell lines. The compounds having cyclopentyl ring are active compared with higher and lower carbon analogues.
中文翻译:

2-(2-(三氟甲基)-6-(取代)咪唑并[1,2-b]哒嗪-3-基)-N-(取代)乙酰胺衍生物的合成和抗增殖活性研究
从3,6-二氯哒嗪合成了一系列新的咪唑并[1,2 - b ]哒嗪-3-基乙酰胺衍生物(9a-9j)。我们已经开发了一种合成功能多样的咪唑的简单策略,并通过一系列步骤报道了哒嗪衍生物。该工作涉及双环咪唑并哒嗪环的形成,卤化,环合,水解,肽偶联和布赫瓦尔德反应。通过IR,1 H NMR,13 C NMR,19确认合成的化合物的结构。F NMR,质谱和元素分析以及纯度通过HPLC检查。通过MTT分析筛选所有合成的化合物对A-549和Du-145癌细胞的抗癌活性。初步的生物测定表明,大多数化合物在不同程度上均表现出抗增殖作用。阿霉素用作阳性对照。合成的化合物在两种细胞系中均显示出IC 50值在1.74μM至16.17μM的范围内。与阿霉素相比,化合物9e,9g和9h在两种细胞系中均具有活性。与较高和较低碳的类似物相比,具有环戊基环的化合物具有活性。
更新日期:2020-02-21
中文翻译:

2-(2-(三氟甲基)-6-(取代)咪唑并[1,2-b]哒嗪-3-基)-N-(取代)乙酰胺衍生物的合成和抗增殖活性研究
从3,6-二氯哒嗪合成了一系列新的咪唑并[1,2 - b ]哒嗪-3-基乙酰胺衍生物(9a-9j)。我们已经开发了一种合成功能多样的咪唑的简单策略,并通过一系列步骤报道了哒嗪衍生物。该工作涉及双环咪唑并哒嗪环的形成,卤化,环合,水解,肽偶联和布赫瓦尔德反应。通过IR,1 H NMR,13 C NMR,19确认合成的化合物的结构。F NMR,质谱和元素分析以及纯度通过HPLC检查。通过MTT分析筛选所有合成的化合物对A-549和Du-145癌细胞的抗癌活性。初步的生物测定表明,大多数化合物在不同程度上均表现出抗增殖作用。阿霉素用作阳性对照。合成的化合物在两种细胞系中均显示出IC 50值在1.74μM至16.17μM的范围内。与阿霉素相比,化合物9e,9g和9h在两种细胞系中均具有活性。与较高和较低碳的类似物相比,具有环戊基环的化合物具有活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号