Separation and Purification Technology ( IF 8.1 ) Pub Date : 2020-02-21 , DOI: 10.1016/j.seppur.2020.116743 Zhanhui Shen , Daoru Liu , Gege Peng , Yuanhao Ma , Jiansheng Li , Jialu Shi , Jianbiao Peng , Linjie Ding
|
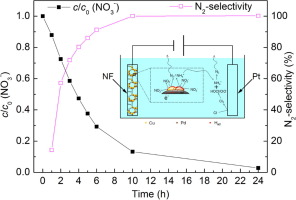
Electrocatalytic reduction of nitrate is a promising technology for nitrate removal from groundwater or surface water to eliminate the negative influence on human. The activity of an electrode usually depends on many factors such as catalytic metal, accessible active centers and electrode geometry. In this work, we prepared Cu/Pd modified nickel foam electrode (Cu/Pd-NF) according to two-step electrodeposition method using nickel foam (NF) as substrate. Cu/Pd particles with a grape-cluster-like shape with pores around 100 nm between clusters were formed on the NF surface according to the field emission scanning electron microscope with energy dispersive spectroscopy (FESEM-EDS) results. The crystal structure and the valence state of the prepared NF-Cu/Pd electrode were analyzed by using powder X-ray diffraction (XRD) and X-ray photoelectron spectroscopy (XPS) technology. Linear sweep voltammetry (LSV) tests indicated that the prepared NF-Cu/Pd electrode had high electrocatalytic activity for nitrate reduction either using Na2SO4 as electrolyte or using NaCl as electrolyte. According to the dynamic experiment, 97% of the initial 100 mg/L NO3--N were removed with a N2-selectivity of 99% at the cathode potential of -1.2 V vs. Ag/AgCl (sat. KCl) when using 0.05 M Na2SO4 as electrolyte. Using NaCl as electrolyte could improve the N2-selectivity of the nitrate electrocatalytic reaction under general conditions. The cathode potential and the current density affects the reaction pathways of nitrate reduction. In the microenvironment around the active sites, the mole ratio of NO2- and Had dominated the main product of NO3- reduction. Long time cyclic voltammetry test indicated that the prepared NF-Cu/Pd electrode has a good long-term electroactive stability for nitrate reduction from water solution. And the prepared NF-Cu/Pd showed high nitrate removal efficiency (96.4%) and N2-selectivity (65.4%) from the municipal wastewater treatment plant (WWTP) effluent.
中文翻译:

使用Cu / Pd改性的Ni泡沫阴极电催化还原水中的硝酸盐:较高的硝酸盐去除效率和N 2选择性
电催化还原硝酸盐是从地下水或地表水中去除硝酸盐以消除对人体的不利影响的有前途的技术。电极的活性通常取决于许多因素,例如催化金属,可及的活性中心和电极几何形状。在这项工作中,我们采用两步电沉积法,以泡沫镍(NF)为基材,制备了铜/钯改性镍泡沫电极(Cu / Pd-NF)。根据具有能量色散光谱的场发射扫描电子显微镜(FESEM-EDS)的结果,在NF表面上形成了具有葡萄簇状的Cu / Pd颗粒,簇之间具有约100nm的孔。用粉末X射线衍射(XRD)和X射线光电子能谱(XPS)技术分析了制备的NF-Cu / Pd电极的晶体结构和价态。线性扫描伏安法(LSV)测试表明,使用Na制备的NF-Cu / Pd电极对硝酸盐还原具有很高的电催化活性2 SO 4作为电解质或使用NaCl作为电解质。根据动态实验,当相对于Ag / AgCl(饱和KCl)的阴极电势为-1.2 V时,以100%/ L的初始NO 3 -- N去除了97%的N 2-选择性为99%。使用0.05 M Na 2 SO 4作为电解质。在一般条件下,使用NaCl作为电解质可以提高硝酸盐电催化反应的N 2-选择性。阴极电势和电流密度影响硝酸盐还原的反应途径。在围绕活性位点微环境,NO的摩尔比2 -和H广告为主NO的主要产物3 -减少。长时间循环伏安法测试表明,所制得的NF-Cu / Pd电极对于从水溶液中还原硝酸盐具有良好的长期电活性稳定性。制备的NF-Cu / Pd对市政污水处理厂废水的硝酸盐去除率高(96.4%),对N 2的选择性高(65.4%)。































 京公网安备 11010802027423号
京公网安备 11010802027423号