当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Reversible Inhibitors of KDM1A Efficacious in Acute Myeloid Leukemia Models.
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-02-13 , DOI: 10.1021/acsmedchemlett.9b00604
Alessia Romussi 1 , Anna Cappa 1 , Paola Vianello 1 , Silvia Brambillasca 1 , Maria Rosaria Cera 1 , Roberto Dal Zuffo 1 , Giovanni Fagà 1 , Raimondo Fattori 1 , Loris Moretti 1 , Paolo Trifirò 1 , Manuela Villa 1 , Stefania Vultaggio 1 , Valentina Cecatiello 2 , Sebastiano Pasqualato 2 , Giulio Dondio 3 , Chi Wai Eric So 4 , Saverio Minucci 1, 5 , Luca Sartori 1 , Mario Varasi 1 , Ciro Mercurio 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-02-13 , DOI: 10.1021/acsmedchemlett.9b00604
Alessia Romussi 1 , Anna Cappa 1 , Paola Vianello 1 , Silvia Brambillasca 1 , Maria Rosaria Cera 1 , Roberto Dal Zuffo 1 , Giovanni Fagà 1 , Raimondo Fattori 1 , Loris Moretti 1 , Paolo Trifirò 1 , Manuela Villa 1 , Stefania Vultaggio 1 , Valentina Cecatiello 2 , Sebastiano Pasqualato 2 , Giulio Dondio 3 , Chi Wai Eric So 4 , Saverio Minucci 1, 5 , Luca Sartori 1 , Mario Varasi 1 , Ciro Mercurio 1
Affiliation
|
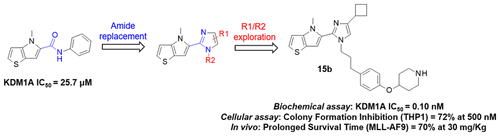
Lysine-specific demethylase 1 (LSD1 or KDM1A) is a FAD-dependent enzyme that acts as a transcription corepressor or coactivator by regulating the methylation status of histone H3 lysines K4 and K9, respectively. KDM1A represents an attractive target for cancer therapy. While, in the past, the main medicinal chemistry strategy toward KDM1A inhibition was based on the optimization of ligands that irreversibly bind the FAD cofactor within the enzyme catalytic site, we and others have also identified reversible inhibitors. Herein we reported the discovery of 5-imidazolylthieno[3,2-b]pyrroles, a new series of KDM1A inhibitors endowed with picomolar inhibitory potency, active in cells and efficacious after oral administration in murine leukemia models.
中文翻译:

发现对急性髓系白血病模型有效的 KDM1A 可逆抑制剂。
赖氨酸特异性去甲基化酶 1(LSD1 或 KDM1A)是一种 FAD 依赖性酶,通过分别调节组蛋白 H3 赖氨酸 K4 和 K9 的甲基化状态,充当转录辅抑制子或共激活子。 KDM1A 代表了癌症治疗的一个有吸引力的靶点。虽然过去抑制 KDM1A 的主要药物化学策略是基于配体的优化,这些配体不可逆地结合酶催化位点内的 FAD 辅因子,但我们和其他人也发现了可逆的抑制剂。在此,我们报告了 5-咪唑基噻吩并[3,2-b]吡咯的发现,这是一系列新的 KDM1A 抑制剂,具有皮摩尔抑制效力,在细胞中具有活性,并且在小鼠白血病模型中口服给药后有效。
更新日期:2020-02-13
中文翻译:

发现对急性髓系白血病模型有效的 KDM1A 可逆抑制剂。
赖氨酸特异性去甲基化酶 1(LSD1 或 KDM1A)是一种 FAD 依赖性酶,通过分别调节组蛋白 H3 赖氨酸 K4 和 K9 的甲基化状态,充当转录辅抑制子或共激活子。 KDM1A 代表了癌症治疗的一个有吸引力的靶点。虽然过去抑制 KDM1A 的主要药物化学策略是基于配体的优化,这些配体不可逆地结合酶催化位点内的 FAD 辅因子,但我们和其他人也发现了可逆的抑制剂。在此,我们报告了 5-咪唑基噻吩并[3,2-b]吡咯的发现,这是一系列新的 KDM1A 抑制剂,具有皮摩尔抑制效力,在细胞中具有活性,并且在小鼠白血病模型中口服给药后有效。

































 京公网安备 11010802027423号
京公网安备 11010802027423号