当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mild Synthesis of Size-Tunable CeO2 Octahedra for Band Gap Variation
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.chemmater.0c00318 Yi-Chun Huang, Shi-Hong Wu, Chien-Hsuan Hsiao, An-Ting Lee, Michael H. Huang
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.chemmater.0c00318 Yi-Chun Huang, Shi-Hong Wu, Chien-Hsuan Hsiao, An-Ting Lee, Michael H. Huang
|
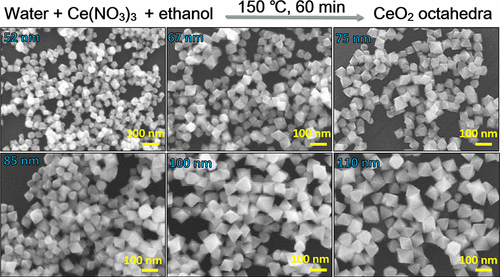
CeO2 octahedral particles with average opposite corner lengths of 52, 67, 75, 85, 100, and 110 nm have been synthesized by heating a water–ethanol mixture of Ce(NO3)3 solution at 150 °C for 1 h. Simply varying the volume of Ce(NO3)3 solution used tunes the particle size. Experimental observations support direct formation of CeO2 from Ce3+ ions. Both light absorption and band-gap-related photoluminescence bands of these CeO2 octahedra red-shift continuously with increasing particle size. Optical band gap varies from 3.42 eV for 52 nm octahedra to 2.94 eV for 110 nm octahedra, so band gap tunability is possible over a very large size range. Mott–Schottky plots were obtained from electrochemical measurements to yield a band diagram of 52, 85, and 110 nm CeO2 octahedra with different valence band and conduction band energies, showing that particle size of semiconductor nanocrystals can significantly tune their band positions. The notable change in valence band positions for the 52 and 110 nm CeO2 octahedra may contribute to their potential difference in electrochemical oxygen evolution reaction activity.
中文翻译:

用于带隙变化的大小可调的CeO 2八面体的温和合成
通过在150°C下加热Ce(NO 3)3溶液的水-乙醇混合物1 h,合成了平均角长分别为52、67、75、85、100和110 nm的CeO 2八面体颗粒。只需改变所用Ce(NO 3)3溶液的体积即可调整粒径。实验观察结果支持由Ce 3+离子直接形成CeO 2。这些CeO 2的光吸收和带隙相关的光致发光带八面体的红移随着粒径的增加而连续变化。光学带隙从52 nm八面体的3.42 eV到110 nm八面体的2.94 eV不等,因此在非常大的尺寸范围内,带隙可调性是可能的。从电化学测量获得Mott–Schottky图,得出具有不同价带和导带能的52、85和110 nm CeO 2八面体的能带图,表明半导体纳米晶体的粒径可以显着调整其能带位置。52和110 nm CeO 2八面体的价带位置的显着变化可能有助于它们在电化学氧释放反应活性中的电势差。
更新日期:2020-03-03
中文翻译:

用于带隙变化的大小可调的CeO 2八面体的温和合成
通过在150°C下加热Ce(NO 3)3溶液的水-乙醇混合物1 h,合成了平均角长分别为52、67、75、85、100和110 nm的CeO 2八面体颗粒。只需改变所用Ce(NO 3)3溶液的体积即可调整粒径。实验观察结果支持由Ce 3+离子直接形成CeO 2。这些CeO 2的光吸收和带隙相关的光致发光带八面体的红移随着粒径的增加而连续变化。光学带隙从52 nm八面体的3.42 eV到110 nm八面体的2.94 eV不等,因此在非常大的尺寸范围内,带隙可调性是可能的。从电化学测量获得Mott–Schottky图,得出具有不同价带和导带能的52、85和110 nm CeO 2八面体的能带图,表明半导体纳米晶体的粒径可以显着调整其能带位置。52和110 nm CeO 2八面体的价带位置的显着变化可能有助于它们在电化学氧释放反应活性中的电势差。































 京公网安备 11010802027423号
京公网安备 11010802027423号