当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Brønsted acidic ionic liquid–catalyzed tandem trimerization of indoles: An efficient approach towards the synthesis of indole 3,3′‐trimers under solvent‐free conditions
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-20 , DOI: 10.1002/jhet.3914 Rana Chatterjee 1 , Sougata Santra 2 , Grigory V. Zyryanov 2, 3 , Adinath Majee 1
Journal of Heterocyclic Chemistry ( IF 2.0 ) Pub Date : 2020-02-20 , DOI: 10.1002/jhet.3914 Rana Chatterjee 1 , Sougata Santra 2 , Grigory V. Zyryanov 2, 3 , Adinath Majee 1
Affiliation
|
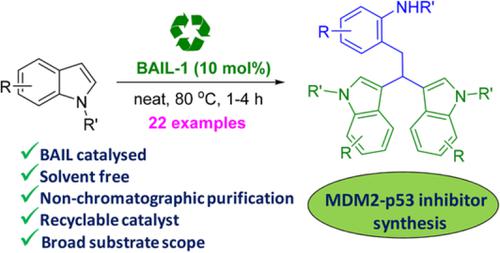
We have observed the role of 1‐butane sulfonic acid‐3‐methylimidazolium tosylate, [BSMIM]OTs, as an organocatalyst for the tandem type trimerization of indoles to synthesize indole 3,3′‐trimers under neat conditions. Using this developed protocol synthesis of indole trimers with various substituted indoles, which are biologically important, has been reported. From the control experiments and literature, a possible mechanism has been proposed via the generation of indolinium cation in the presence of the ionic liquid catalyst. The catalyst has been recycled for several times. The significant advantages of our methodology are clean reaction with very short time, no chromatography for purification, commercially available substrates, neat reaction conditions, and in the absence of metal. Using the protocol, the MDM2‐p53 inhibitor has been synthesized in gram scale with high yield.
中文翻译:

布朗斯台德酸性离子液体催化的吲哚串联三聚:在无溶剂条件下合成吲哚3,3'-三聚体的有效方法
我们已经观察到1-丁烷磺酸-3-甲基咪唑鎓甲苯磺酸盐[BSMIM] OTs在纯净条件下作为吲哚串联型三聚反应有机化合物合成吲哚3,3'-三聚体的作用。已经报道了使用这种开发的方案合成具有生物学重要性的各种取代的吲哚的吲哚三聚体。从对照实验和文献中,已经提出了通过在离子液体催化剂存在下生成吲哚阳离子的可能机理。催化剂已循环使用了几次。我们方法的显着优势是反应时间短,反应纯净,无需纯化即可进行色谱分离,可购得的底物,反应条件纯净且无金属。使用协议,
更新日期:2020-02-20
中文翻译:

布朗斯台德酸性离子液体催化的吲哚串联三聚:在无溶剂条件下合成吲哚3,3'-三聚体的有效方法
我们已经观察到1-丁烷磺酸-3-甲基咪唑鎓甲苯磺酸盐[BSMIM] OTs在纯净条件下作为吲哚串联型三聚反应有机化合物合成吲哚3,3'-三聚体的作用。已经报道了使用这种开发的方案合成具有生物学重要性的各种取代的吲哚的吲哚三聚体。从对照实验和文献中,已经提出了通过在离子液体催化剂存在下生成吲哚阳离子的可能机理。催化剂已循环使用了几次。我们方法的显着优势是反应时间短,反应纯净,无需纯化即可进行色谱分离,可购得的底物,反应条件纯净且无金属。使用协议,






























 京公网安备 11010802027423号
京公网安备 11010802027423号