当前位置:
X-MOL 学术
›
Sci. Total Environ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective adsorption mechanisms of pharmaceuticals on benzene-1,4-dicarboxylic acid-based MOFs: Effects of a flexible framework, adsorptive interactions and the DFT study.
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.scitotenv.2020.137449 Dujduan Sompornpailin 1 , Chalita Ratanatawanate 2 , Chanchai Sattayanon 3 , Supawadee Namuangruk 3 , Patiparn Punyapalakul 4
Science of the Total Environment ( IF 8.2 ) Pub Date : 2020-02-20 , DOI: 10.1016/j.scitotenv.2020.137449 Dujduan Sompornpailin 1 , Chalita Ratanatawanate 2 , Chanchai Sattayanon 3 , Supawadee Namuangruk 3 , Patiparn Punyapalakul 4
Affiliation
|
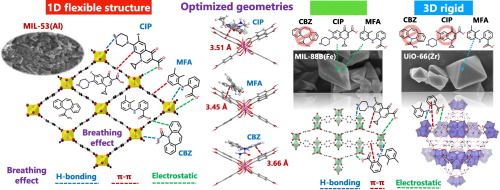
The synergetic effects of benzene-1,4-dicarboxylic acid (BDC) linker structure and the metal cluster of MOFs on adsorption mechanisms of carbamazepine, ciprofloxacin and mefenamic acid were investigated in single and mixed solutions. A 1D flexible framework MIL-53(Al), 3D rigid framework UiO-66(Zr) and 3D flexible framework MIL-88B(Fe) were applied as adsorbents. The breathing effect of MIL-53(Al) caused by its flexible structure can enhance intraparticle diffusion for all pharmaceuticals and perform a critical role in excellent adsorption performances. The 3D rigid BDC structure of UiO-66(Zr) caused a steric effect that reflected low or negligible adsorption. Unless concerning accessibility through the internal structure of the MOFs, the binding strengths calculated by the DFT study were in the following order: MIL-88B(Fe) > MIL-53(Al) > UiO-66(Zr). The Fe cluster in MIL-88B(Fe) seems to have the highest affinity for the carboxylic group of pharmaceuticals compared with Al and Zr; however, the lower porosity of MIL-88B(Fe) might limit the adsorption capacity. Moreover, in mixed solutions, the higher acidity of mefenamic acid can enhance competitive performance in interactions with the metal cation cluster of each MOF. Together with the breathing effect, H-bonding and π-π interaction were shown to be the alternative interactions of synergetic adsorption mechanisms.
中文翻译:

药物在基于苯-1,4-二羧酸的MOF上的选择性吸附机制:柔性框架,吸附相互作用和DFT研究的影响。
研究了单,混合溶液中苯-1,4-二羧酸(BDC)连接基结构和MOFs金属簇对卡马西平,环丙沙星和甲芬那酸吸附机理的协同作用。一维柔性框架MIL-53(Al),3D刚性框架UiO-66(Zr)和3D柔性框架MIL-88B(Fe)被用作吸附剂。MIL-53(Al)的柔性结构引起的呼吸作用可增强所有药物的颗粒内扩散,并在出色的吸附性能中发挥关键作用。UiO-66(Zr)的3D刚性BDC结构引起空间效应,反映了低或可忽略的吸附。除非涉及通过MOF内部结构的可及性,否则DFT研究计算出的结合强度按以下顺序排列:MIL-88B(Fe)> MIL-53(Al)> UiO-66(Zr)。与Al和Zr相比,MIL-88B(Fe)中的Fe簇对药物的羧基具有最高的亲和力;但是,MIL-88B(Fe)的较低孔隙率可能会限制其吸附能力。此外,在混合溶液中,较高的甲芬那酸酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。甲芬那酸的较高酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。甲芬那酸的较高酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。
更新日期:2020-02-20
中文翻译:

药物在基于苯-1,4-二羧酸的MOF上的选择性吸附机制:柔性框架,吸附相互作用和DFT研究的影响。
研究了单,混合溶液中苯-1,4-二羧酸(BDC)连接基结构和MOFs金属簇对卡马西平,环丙沙星和甲芬那酸吸附机理的协同作用。一维柔性框架MIL-53(Al),3D刚性框架UiO-66(Zr)和3D柔性框架MIL-88B(Fe)被用作吸附剂。MIL-53(Al)的柔性结构引起的呼吸作用可增强所有药物的颗粒内扩散,并在出色的吸附性能中发挥关键作用。UiO-66(Zr)的3D刚性BDC结构引起空间效应,反映了低或可忽略的吸附。除非涉及通过MOF内部结构的可及性,否则DFT研究计算出的结合强度按以下顺序排列:MIL-88B(Fe)> MIL-53(Al)> UiO-66(Zr)。与Al和Zr相比,MIL-88B(Fe)中的Fe簇对药物的羧基具有最高的亲和力;但是,MIL-88B(Fe)的较低孔隙率可能会限制其吸附能力。此外,在混合溶液中,较高的甲芬那酸酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。甲芬那酸的较高酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。甲芬那酸的较高酸度可增强与每个MOF的金属阳离子簇相互作用时的竞争性能。除呼吸作用外,氢键和π-π相互作用被证明是协同吸附机制的另一种相互作用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号