当前位置:
X-MOL 学术
›
Spectrochim. Acta. A Mol. Biomol. Spectrosc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The interaction mechanism between fludarabine and human serum albumin researched by comprehensive spectroscopic methods and molecular docking technique.
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.saa.2020.118170
XiaoLe Han 1 , Hao Hao 1 , QingYu Li 1 , ChenYin Liu 1 , JiaWen Lei 1 , Fan Yu 1 , Ke Chen 1 , Yi Liu 2 , Tao Huang 1
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy ( IF 4.3 ) Pub Date : 2020-02-19 , DOI: 10.1016/j.saa.2020.118170
XiaoLe Han 1 , Hao Hao 1 , QingYu Li 1 , ChenYin Liu 1 , JiaWen Lei 1 , Fan Yu 1 , Ke Chen 1 , Yi Liu 2 , Tao Huang 1
Affiliation
|
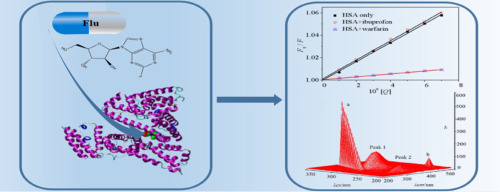
Fludarabine (Flu) is widely used to treat B-cell chronic lymphocytic leukemia. HSA is of the essence to human, especially in blood circulation system. The interaction mechanism between Flu and HSA was studied by comprehensive spectroscopic methods and molecular docking technique. UV-vis and FL spectrum results indicated that Flu bond with HSA, and there was a new complex produced at the binding site I in subdomain IIA. Association constants at 298 K were 1.637 × 104 M-1 and 1.552 × 104 M-1 at 310 K, respectively. The negative enthalpy (ΔH) and positive entropy (ΔS) values for the interaction revealed that the binding behavior was driven by hydrophobic forces and hydrogen bonds. The results obtained from UV, RLS spectra, 3D fluorescence and CD spectrum illustrated that Flu could change the secondary structure of HSA. According to molecule docking result, the binding energy of interaction is -11.15 kcal/mol.
中文翻译:

综合光谱法和分子对接技术研究了氟达拉滨与人血清白蛋白的相互作用机理。
氟达拉滨(Flu)被广泛用于治疗B细胞慢性淋巴细胞性白血病。HSA对人体至关重要,尤其是在血液循环系统中。通过综合光谱学方法和分子对接技术研究了流感病毒与HSA之间的相互作用机理。UV-vis和FL光谱结果表明Flu与HSA键合,并且在亚结构域IIA的结合位点I处产生了新的复合物。298 K时的缔合常数分别为310 K时的1.637×104 M-1和1.552×104 M-1。相互作用的负焓(ΔH)和正熵(ΔS)值表明,结合行为是由疏水力和氢键驱动的。从紫外线,RLS光谱,3D荧光和CD光谱获得的结果表明,流感可以改变HSA的二级结构。
更新日期:2020-02-20
中文翻译:

综合光谱法和分子对接技术研究了氟达拉滨与人血清白蛋白的相互作用机理。
氟达拉滨(Flu)被广泛用于治疗B细胞慢性淋巴细胞性白血病。HSA对人体至关重要,尤其是在血液循环系统中。通过综合光谱学方法和分子对接技术研究了流感病毒与HSA之间的相互作用机理。UV-vis和FL光谱结果表明Flu与HSA键合,并且在亚结构域IIA的结合位点I处产生了新的复合物。298 K时的缔合常数分别为310 K时的1.637×104 M-1和1.552×104 M-1。相互作用的负焓(ΔH)和正熵(ΔS)值表明,结合行为是由疏水力和氢键驱动的。从紫外线,RLS光谱,3D荧光和CD光谱获得的结果表明,流感可以改变HSA的二级结构。

































 京公网安备 11010802027423号
京公网安备 11010802027423号