当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High Selective Catalyst for Ethylene Epoxidation to Ethylene Oxide: A DFT Investigation
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.apsusc.2020.145799 Sarawoot Impeng , Thantip Roongcharoen , Phornphimon Maitarad , Hongmin Wu , Chirawat Chitpakdee , Vinich Promarak , Liyi Shi , Supawadee Namuangruk
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.apsusc.2020.145799 Sarawoot Impeng , Thantip Roongcharoen , Phornphimon Maitarad , Hongmin Wu , Chirawat Chitpakdee , Vinich Promarak , Liyi Shi , Supawadee Namuangruk
|
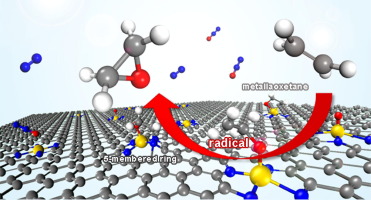
Abstract Ethylene epoxidation to produce ethylene oxide is crucial in both fundamental knowledge and industrial chemical process. The reaction mechanism of ethylene epoxidation with N2O catalyzed by Mn-coordinated porphyrin-like graphene (Mn-N4GP) was investigated using dispersion-corrected density functional theory calculations. The reaction proceeds through two consecutive steps, (1) Mn O active site (MnO-N4GP) formation, followed by (2) ethylene epoxidation on the Mn O. The first step is a feasible process with the energy barrier of 0.77 eV and the MnO-N4GP is thermodynamically stable. The ethylene epoxidation in the second step competitively can undergo three possible intermediate-pathways; carboradical, alkoxide radical, and manganaoxetane intermediates. By systematic study of all possible pathways, we found that the pathway for alkoxide radical intermediate shows the most feasibility which it subsequently converts to three competitive products with the energy barriers of 0.25 eV, 0.56 eV, and 0.46 eV for the formation of ethylene oxide, acetaldehyde, and 5-membered ring (5MR) species, respectively. This catalyst is remarkably selective to ethylene oxide by 105 and 104 times compared with acetaldehyde and 5MR side products, respectively. Thus, the Mn-N4GP catalyst is suggested as a promising catalyst in terms of activity and selectivity for ethylene epoxidation using N2O as an oxidizing agent in mild condition.
中文翻译:

用于乙烯环氧化生成环氧乙烷的高选择性催化剂:DFT 研究
摘要 乙烯环氧化生产环氧乙烷在基础知识和工业化学过程中都至关重要。使用色散校正密度泛函理论计算研究了锰配位卟啉类石墨烯 (Mn-N4GP) 催化乙烯与 N2O 环氧化的反应机理。该反应通过两个连续的步骤进行,(1) Mn O 活性位点 (MnO-N4GP) 的形成,然后是 (2) Mn O 上的乙烯环氧化。第一步是一个可行的过程,能量势垒为 0.77 eV,并且MnO-N4GP 是热力学稳定的。第二步中的乙烯竞争性环氧化可经历三种可能的中间途径;碳自由基、醇盐自由基和锰氧杂环丁烷中间体。通过对所有可能途径的系统研究,我们发现醇盐自由基中间体的途径显示出最可行的途径,它随后转化为三种竞争产物,其能垒分别为 0.25 eV、0.56 eV 和 0.46 eV,用于形成环氧乙烷、乙醛和五元环( 5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。和 5 元环 (5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。和 5 元环 (5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。
更新日期:2020-05-01
中文翻译:

用于乙烯环氧化生成环氧乙烷的高选择性催化剂:DFT 研究
摘要 乙烯环氧化生产环氧乙烷在基础知识和工业化学过程中都至关重要。使用色散校正密度泛函理论计算研究了锰配位卟啉类石墨烯 (Mn-N4GP) 催化乙烯与 N2O 环氧化的反应机理。该反应通过两个连续的步骤进行,(1) Mn O 活性位点 (MnO-N4GP) 的形成,然后是 (2) Mn O 上的乙烯环氧化。第一步是一个可行的过程,能量势垒为 0.77 eV,并且MnO-N4GP 是热力学稳定的。第二步中的乙烯竞争性环氧化可经历三种可能的中间途径;碳自由基、醇盐自由基和锰氧杂环丁烷中间体。通过对所有可能途径的系统研究,我们发现醇盐自由基中间体的途径显示出最可行的途径,它随后转化为三种竞争产物,其能垒分别为 0.25 eV、0.56 eV 和 0.46 eV,用于形成环氧乙烷、乙醛和五元环( 5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。和 5 元环 (5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。和 5 元环 (5MR) 物种,分别。该催化剂对环氧乙烷的选择性分别是乙醛和 5MR 副产物的 105 和 104 倍。因此,就在温和条件下使用 N2O 作为氧化剂的乙烯环氧化的活性和选择性而言,Mn-N4GP 催化剂被认为是一种有前途的催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号