Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Surfactant-Keratin Hydrolysate Interactions on the Hydration Properties of a Stratum Corneum Substitute.
Langmuir ( IF 3.7 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.langmuir.0c00265 Prasad Nithianandam 1 , Saikat Das 2 , Yoonjee C Park 1, 2
Langmuir ( IF 3.7 ) Pub Date : 2020-02-20 , DOI: 10.1021/acs.langmuir.0c00265 Prasad Nithianandam 1 , Saikat Das 2 , Yoonjee C Park 1, 2
Affiliation
|
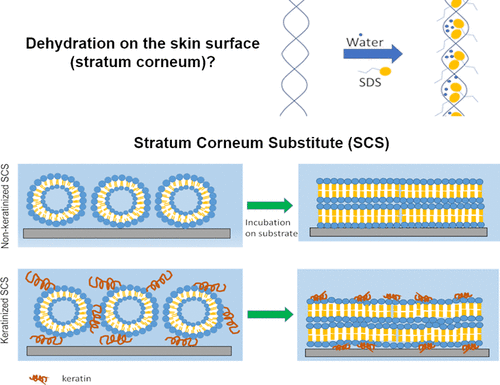
A novel stratum corneum substitute (SCS) has been developed and fundamental mechanism of dehydration process has been studied using the SCS. After washing with cleansers which contain surfactants, our skin 'feels' dehydrated (or hydrated). Although many studies focused on effect of surfactants on regulation of the water loss by the lipid bilayers in SC for a long time scale or at equilibrium, little study has focused on the acute effect of surfactant interaction on dehydration. In addition, interaction between surfactant and keratin has been often underappreciated compared to lipid bilayers although keratin is the major non-aqueous component of SC. Here we have developed novel SCS models, non-keratinized (lipid only) and keratinized, to study the effect of keratin on dehydration rate. We have confirmed that the lipid organizational structure of the SCS was similar to the human SC's using x-ray scattering. We have revealed that keratin plays a significant role in dehydration rate, accelerating the rate for the short-term. We also have demonstrated the effect of surfactant on dehydration is more pronounced for keratinized samples than one for the non-keratinized sample. However, the dehydration rate for the non-keratinized SCS with surfactant became faster than the one for the keratinized SCS after 20 minutes of evaporation process, suggesting that water binding sites of keratin slowed down evaporation while the surfactant interacting with the lipids accelerated water loss. Lastly, the study demonstrated that the SCS model can be a great platform to test macroscopic properties and analyze the underlying mechanism at the molecular level for various chemicals.
中文翻译:

表面活性剂-角蛋白水解产物相互作用对角质层替代物水化性质的影响。
已经开发了一种新颖的角质层替代物(SCS),并且已经使用SCS研究了脱水过程的基本机理。用含有表面活性剂的清洁剂洗涤后,我们的皮肤“感觉”脱水(或水合)。尽管许多研究集中于表面活性剂对SC脂质双分子层在较长时间内或处于平衡状态下失水调节的影响,但很少有研究集中于表面活性剂相互作用对脱水的急性影响。此外,尽管角蛋白是SC的主要非水成分,但与脂质双层相比,表面活性剂与角蛋白之间的相互作用常常被低估了。在这里,我们开发了新颖的SCS模型(非角化(仅脂质)和角化),以研究角蛋白对脱水率的影响。我们已经证实,使用X射线散射,SCS的脂质组织结构与人类SC相似。我们发现角蛋白在脱水率中起着重要作用,短期内会加快脱水率。我们还证明,对于角化样品,表面活性剂对脱水的影响比未角化样品对脱水的影响更为明显。然而,蒸发20分钟后,具有表面活性剂的非角化SCS的脱水速率变得比角化SCS的脱水速率快,这表明角蛋白的水结合位点减慢了蒸发速度,而表面活性剂与脂质相互作用加速了失水。最后,
更新日期:2020-03-09
中文翻译:

表面活性剂-角蛋白水解产物相互作用对角质层替代物水化性质的影响。
已经开发了一种新颖的角质层替代物(SCS),并且已经使用SCS研究了脱水过程的基本机理。用含有表面活性剂的清洁剂洗涤后,我们的皮肤“感觉”脱水(或水合)。尽管许多研究集中于表面活性剂对SC脂质双分子层在较长时间内或处于平衡状态下失水调节的影响,但很少有研究集中于表面活性剂相互作用对脱水的急性影响。此外,尽管角蛋白是SC的主要非水成分,但与脂质双层相比,表面活性剂与角蛋白之间的相互作用常常被低估了。在这里,我们开发了新颖的SCS模型(非角化(仅脂质)和角化),以研究角蛋白对脱水率的影响。我们已经证实,使用X射线散射,SCS的脂质组织结构与人类SC相似。我们发现角蛋白在脱水率中起着重要作用,短期内会加快脱水率。我们还证明,对于角化样品,表面活性剂对脱水的影响比未角化样品对脱水的影响更为明显。然而,蒸发20分钟后,具有表面活性剂的非角化SCS的脱水速率变得比角化SCS的脱水速率快,这表明角蛋白的水结合位点减慢了蒸发速度,而表面活性剂与脂质相互作用加速了失水。最后,


















































 京公网安备 11010802027423号
京公网安备 11010802027423号