当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prevention of aspartimide formation during peptide synthesis using cyanosulfurylides as carboxylic acid-protecting groups.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-20 , DOI: 10.1038/s41467-020-14755-6 Kevin Neumann 1 , Jakob Farnung 1 , Simon Baldauf 1 , Jeffrey W Bode 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-20 , DOI: 10.1038/s41467-020-14755-6 Kevin Neumann 1 , Jakob Farnung 1 , Simon Baldauf 1 , Jeffrey W Bode 1, 2
Affiliation
|
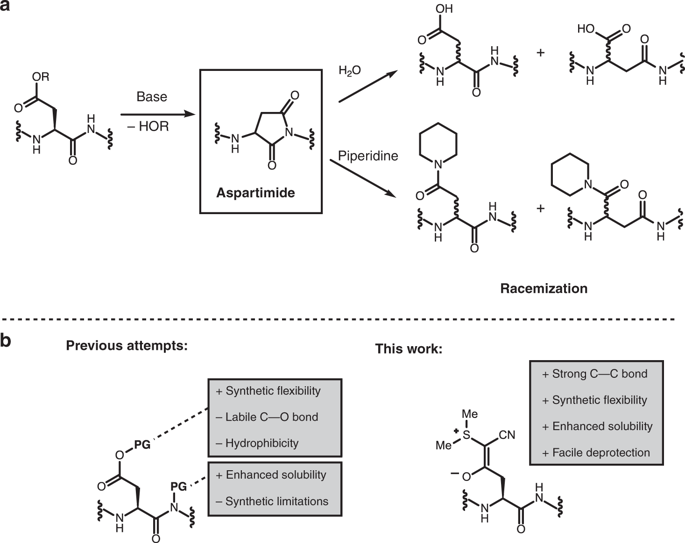
Although peptide chemistry has made great progress, the frequent occurrence of aspartimide formation during peptide synthesis remains a formidable challenge. Aspartimide formation leads to low yields in addition to costly purification or even inaccessible peptide sequences. Here, we report an alternative approach to address this longstanding challenge of peptide synthesis by utilizing cyanosulfurylides to mask carboxylic acids by a stable C-C bond. These functional groups-formally zwitterionic species-are exceptionally stable to all common manipulations and impart improved solubility during synthesis. Deprotection is readily and rapidly achieved under aqueous conditions with electrophilic halogenating agents via a highly selective C-C bond cleavage reaction. This protecting group is employed for the synthesis of a range of peptides and proteins including teduglutide, ubiquitin, and the low-density lipoprotein class A. This protecting group strategy has the potential to overcome one of the most difficult aspects of modern peptide chemistry.
中文翻译:

使用氰基硫酰化物作为羧酸保护基团,防止肽合成过程中天冬酰亚胺的形成。
尽管肽化学已经取得了长足的进步,但肽合成过程中频繁发生的天冬酰亚胺形成仍然是一个艰巨的挑战。天冬酰亚胺的形成除了成本高昂之外还导致产量低,甚至导致肽序列难以接近。在这里,我们报告了一种替代方法来解决肽合成的长期挑战,即利用氰基硫酰化物通过稳定的 CC 键掩蔽羧酸。这些官能团(正式为两性离子物质)对于所有常见操作都非常稳定,并在合成过程中提高了溶解度。在水性条件下,使用亲电卤化剂通过高选择性的 CC 键断裂反应可以轻松快速地实现脱保护。该保护基用于合成一系列肽和蛋白质,包括替度鲁肽、泛素和 A 类低密度脂蛋白。该保护基策略有可能克服现代肽化学中最困难的方面之一。
更新日期:2020-02-20
中文翻译:

使用氰基硫酰化物作为羧酸保护基团,防止肽合成过程中天冬酰亚胺的形成。
尽管肽化学已经取得了长足的进步,但肽合成过程中频繁发生的天冬酰亚胺形成仍然是一个艰巨的挑战。天冬酰亚胺的形成除了成本高昂之外还导致产量低,甚至导致肽序列难以接近。在这里,我们报告了一种替代方法来解决肽合成的长期挑战,即利用氰基硫酰化物通过稳定的 CC 键掩蔽羧酸。这些官能团(正式为两性离子物质)对于所有常见操作都非常稳定,并在合成过程中提高了溶解度。在水性条件下,使用亲电卤化剂通过高选择性的 CC 键断裂反应可以轻松快速地实现脱保护。该保护基用于合成一系列肽和蛋白质,包括替度鲁肽、泛素和 A 类低密度脂蛋白。该保护基策略有可能克服现代肽化学中最困难的方面之一。











































 京公网安备 11010802027423号
京公网安备 11010802027423号