当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PUMA-mediated epithelial cell apoptosis promotes Helicobacter pylori infection-mediated gastritis.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-02-20 , DOI: 10.1038/s41419-020-2339-x Yini Dang 1 , Yifeng Zhang 2 , Lingyan Xu 3 , Xiaoying Zhou 1 , Yanhong Gu 3 , Jian Yu 4 , Shidai Jin 3 , Haoming Ji 5 , Yongqian Shu 3 , Guoxin Zhang 1 , Shiyun Cui 3 , Jing Sun 3
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-02-20 , DOI: 10.1038/s41419-020-2339-x Yini Dang 1 , Yifeng Zhang 2 , Lingyan Xu 3 , Xiaoying Zhou 1 , Yanhong Gu 3 , Jian Yu 4 , Shidai Jin 3 , Haoming Ji 5 , Yongqian Shu 3 , Guoxin Zhang 1 , Shiyun Cui 3 , Jing Sun 3
Affiliation
|
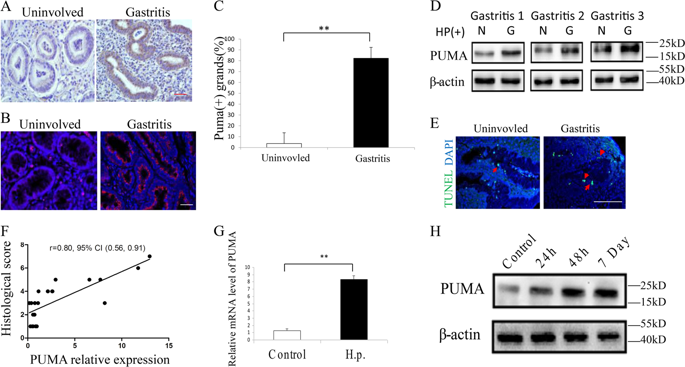
The molecular mechanism responsible for Helicobacter pylori infection-mediated gastritis and carcinogenesis is not yet clear. Increased evidence suggests that chronic gastritis and elevated gastric epithelial cell (GEC) apoptosis are crucial events during stomach carcinoma transformation. PUMA is a potent proapoptotic Bcl-2 protein and mediates acute tissue injury. In this study, we aimed to investigate the role of PUMA in GEC apoptosis and inflammation induced by H. pylori infection. As a result, we found that PUMA expression was elevated in gastritis tissues compared with uninvolved tissues, and it was correlated with the severity of apoptosis and gastritis. In mice, PUMA mRNA and protein were markedly induced in GECs upon induction of gastritis by H. pylori. PUMA-deficient mice were highly resistant to apoptosis and gastritis induced by H. pylori. Furthermore, the transcription factor NF-κB p65 binds to PUMA promoter to activate PUMA transcription after H. pylori infection. In addition, NF-κB inhibitor could rescue H. pylori-induced apoptosis and gastritis. Finally, H. pylori-induced activation of p-p65 and PUMA was mediated via Toll-like receptor 2 (TLR2) and blocked in TLR2 knockout mice. Taken together, these results verified the pro-inflammatory effect of PUMA in H. pylori-infected gastric tissue. Moreover, TLR2/NF-κB-mediated transcriptional regulation of PUMA contributes to the pathogenesis of H. pylori-infected gastritis.
中文翻译:

PUMA介导的上皮细胞凋亡促进幽门螺杆菌感染介导的胃炎。
幽门螺杆菌感染介导的胃炎和致癌作用的分子机制尚不清楚。越来越多的证据表明,慢性胃炎和胃上皮细胞(GEC)凋亡升高是胃癌转化过程中的关键事件。PUMA是一种有效的促凋亡Bcl-2蛋白,可介导急性组织损伤。在这项研究中,我们旨在研究PUMA在幽门螺杆菌感染诱导的GEC细胞凋亡和炎症中的作用。结果,我们发现与未累及的组织相比,PUMA在胃炎组织中的表达升高,并且与细胞凋亡和胃炎的严重程度相关。在小鼠中,幽门螺杆菌诱导胃炎后,GEC中会明显诱导PUMA mRNA和蛋白。缺乏PUMA的小鼠对H诱导的细胞凋亡和胃炎高度耐药。幽门螺旋杆菌。此外,在幽门螺杆菌感染后,转录因子NF-κBp65与PUMA启动子结合以激活PUMA转录。此外,NF-κB抑制剂可以挽救幽门螺杆菌诱导的细胞凋亡和胃炎。最后,幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。
更新日期:2020-02-20
中文翻译:

PUMA介导的上皮细胞凋亡促进幽门螺杆菌感染介导的胃炎。
幽门螺杆菌感染介导的胃炎和致癌作用的分子机制尚不清楚。越来越多的证据表明,慢性胃炎和胃上皮细胞(GEC)凋亡升高是胃癌转化过程中的关键事件。PUMA是一种有效的促凋亡Bcl-2蛋白,可介导急性组织损伤。在这项研究中,我们旨在研究PUMA在幽门螺杆菌感染诱导的GEC细胞凋亡和炎症中的作用。结果,我们发现与未累及的组织相比,PUMA在胃炎组织中的表达升高,并且与细胞凋亡和胃炎的严重程度相关。在小鼠中,幽门螺杆菌诱导胃炎后,GEC中会明显诱导PUMA mRNA和蛋白。缺乏PUMA的小鼠对H诱导的细胞凋亡和胃炎高度耐药。幽门螺旋杆菌。此外,在幽门螺杆菌感染后,转录因子NF-κBp65与PUMA启动子结合以激活PUMA转录。此外,NF-κB抑制剂可以挽救幽门螺杆菌诱导的细胞凋亡和胃炎。最后,幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。幽门螺杆菌诱导的p-p65和PUMA激活是通过Toll样受体2(TLR2)介导的,并在TLR2基因敲除小鼠中被阻断。综上所述,这些结果证实了PUMA在幽门螺杆菌感染的胃组织中的促炎作用。而且,TLR2 /NF-κB介导的PUMA转录调控有助于幽门螺杆菌感染的胃炎的发病机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号