当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric N-sulfonyl amide C-N bond activation to access axially chiral biaryl amino acids.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-19 , DOI: 10.1038/s41467-020-14799-8 Guanjie Wang 1 , Qianqian Shi 2 , Wanyao Hu 1 , Tao Chen 1 , Yingying Guo 1 , Zhouli Hu 1 , Minghua Gong 1 , Jingcheng Guo 1 , Donghui Wei 2 , Zhenqian Fu 1, 3 , Wei Huang 1, 3
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-19 , DOI: 10.1038/s41467-020-14799-8 Guanjie Wang 1 , Qianqian Shi 2 , Wanyao Hu 1 , Tao Chen 1 , Yingying Guo 1 , Zhouli Hu 1 , Minghua Gong 1 , Jingcheng Guo 1 , Donghui Wei 2 , Zhenqian Fu 1, 3 , Wei Huang 1, 3
Affiliation
|
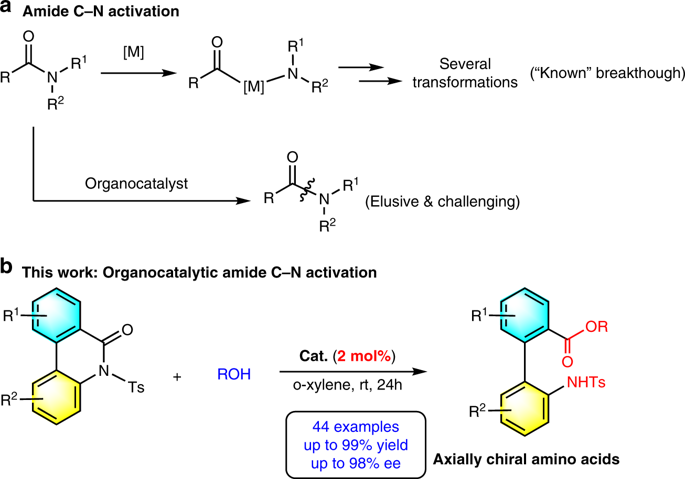
Amides are among the most fundamental functional groups and essential structural units, widely used in chemistry, biochemistry and material science. Amide synthesis and transformations is a topic of continuous interest in organic chemistry. However, direct catalytic asymmetric activation of amide C-N bonds still remains a long-standing challenge due to high stability of amide linkages. Herein, we describe an organocatalytic asymmetric amide C-N bonds cleavage of N-sulfonyl biaryl lactams under mild conditions, developing a general and practical method for atroposelective construction of axially chiral biaryl amino acids. A structurally diverse set of axially chiral biaryl amino acids are obtained in high yields with excellent enantioselectivities. Moreover, a variety of axially chiral unsymmetrical biaryl organocatalysts are efficiently constructed from the resulting axially chiral biaryl amino acids by our present strategy, and show competitive outcomes in asymmetric reactions.
中文翻译:

有机催化不对称 N-磺酰酰胺 CN 键活化以获得轴向手性联芳基氨基酸。
酰胺是最基本的官能团和必要的结构单元,广泛应用于化学、生物化学和材料科学。酰胺合成和转化是有机化学中持续关注的主题。然而,由于酰胺键的高稳定性,酰胺CN键的直接催化不对称活化仍然是一个长期存在的挑战。在此,我们描述了在温和条件下N-磺酰基联芳基内酰胺的有机催化不对称酰胺CN键断裂,开发了一种用于轴手性联芳基氨基酸的逆向选择性构建的通用且实用的方法。以高产率获得了一组结构多样的轴向手性联芳基氨基酸,并具有优异的对映选择性。此外,通过我们目前的策略,由所得的轴向手性联芳基氨基酸有效地构建了多种轴向手性不对称联芳基有机催化剂,并在不对称反应中显示出有竞争力的结果。
更新日期:2020-02-19
中文翻译:

有机催化不对称 N-磺酰酰胺 CN 键活化以获得轴向手性联芳基氨基酸。
酰胺是最基本的官能团和必要的结构单元,广泛应用于化学、生物化学和材料科学。酰胺合成和转化是有机化学中持续关注的主题。然而,由于酰胺键的高稳定性,酰胺CN键的直接催化不对称活化仍然是一个长期存在的挑战。在此,我们描述了在温和条件下N-磺酰基联芳基内酰胺的有机催化不对称酰胺CN键断裂,开发了一种用于轴手性联芳基氨基酸的逆向选择性构建的通用且实用的方法。以高产率获得了一组结构多样的轴向手性联芳基氨基酸,并具有优异的对映选择性。此外,通过我们目前的策略,由所得的轴向手性联芳基氨基酸有效地构建了多种轴向手性不对称联芳基有机催化剂,并在不对称反应中显示出有竞争力的结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号