当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral 1,5-disubstituted 1,2,3-triazoles - versatile tools for foldamers and peptidomimetic applications.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-02-26 , DOI: 10.1039/d0ob00168f Anna Said Stålsmeden 1 , Andrew J Paterson 1 , Imola Cs Szigyártó 2 , Linda Thunberg 3 , Johan R Johansson 4 , Tamás Beke-Somfai 2 , Nina Kann 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-02-26 , DOI: 10.1039/d0ob00168f Anna Said Stålsmeden 1 , Andrew J Paterson 1 , Imola Cs Szigyártó 2 , Linda Thunberg 3 , Johan R Johansson 4 , Tamás Beke-Somfai 2 , Nina Kann 1
Affiliation
|
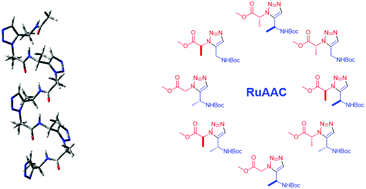
1,4- and 1,5-Disubstituted triazole amino acid monomers have gained increasing interest among peptidic foldamers, as they are easily prepared via Cu- and Ru-catalyzed click reactions, with the potential for side chain variation. While the latter is key to their applicability, the synthesis and structural properties of the chiral mono- or disubstituted triazole amino acids have only been partially addressed. We here present the synthesis of all eight possible chiral derivatives of a triazole monomer prepared via a ruthenium-catalyzed azide alkyne cycloaddition (RuAAC). To evaluate the conformational properties of the individual building units, a systematic quantum chemical study was performed on all monomers, indicating their capacity to form several low energy conformers. This feature may be used to effect structural diversity when the monomers are inserted into various peptide sequences. We envisage that these results will facilitate new applications for these artificial oligomeric compounds in diverse areas, ranging from pharmaceutics to biotechnology.
中文翻译:

手性1,5-二取代1,2,3-三唑-用于折叠剂和拟肽应用的多功能工具。
1,4-和1,5-二取代三唑氨基酸单体越来越受到肽折叠剂的关注,因为它们很容易通过Cu和Ru催化的点击反应制备,具有侧链变化的潜力。尽管后者是其适用性的关键,但手性单或双取代三唑氨基酸的合成和结构性质仅得到部分解决。我们在这里介绍了通过钌催化的叠氮化物炔烃环加成(RuAAC)制备的三唑单体的所有八种可能的手性衍生物的合成。为了评估各个构建单元的构象性质,对所有单体进行了系统的量子化学研究,表明它们形成几种低能构象异构体的能力。当单体插入各种肽序列时,该特征可用于实现结构多样性。我们设想,这些结果将促进这些人工寡聚化合物在从制药到生物技术等各个领域的新应用。
更新日期:2020-03-12
中文翻译:

手性1,5-二取代1,2,3-三唑-用于折叠剂和拟肽应用的多功能工具。
1,4-和1,5-二取代三唑氨基酸单体越来越受到肽折叠剂的关注,因为它们很容易通过Cu和Ru催化的点击反应制备,具有侧链变化的潜力。尽管后者是其适用性的关键,但手性单或双取代三唑氨基酸的合成和结构性质仅得到部分解决。我们在这里介绍了通过钌催化的叠氮化物炔烃环加成(RuAAC)制备的三唑单体的所有八种可能的手性衍生物的合成。为了评估各个构建单元的构象性质,对所有单体进行了系统的量子化学研究,表明它们形成几种低能构象异构体的能力。当单体插入各种肽序列时,该特征可用于实现结构多样性。我们设想,这些结果将促进这些人工寡聚化合物在从制药到生物技术等各个领域的新应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号