当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Bimetallic Fe-Mn Oxide-Activated Oxone for In Situ Chemical Oxidation (ISCO) of Trichloroethylene in Groundwater: Efficiency, Sustained Activity, and Mechanism Investigation.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.est.0c00151 Xueying Yang 1 , Jingsheng Cai 2 , Xiaoning Wang 1 , Yifan Li 3 , Zhangxiong Wu 1 , Winston Duo Wu 1 , Xiao Dong Chen 1 , Jingyu Sun 2 , Sheng-Peng Sun 1 , Zhaohui Wang 4, 5
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-18 , DOI: 10.1021/acs.est.0c00151 Xueying Yang 1 , Jingsheng Cai 2 , Xiaoning Wang 1 , Yifan Li 3 , Zhangxiong Wu 1 , Winston Duo Wu 1 , Xiao Dong Chen 1 , Jingyu Sun 2 , Sheng-Peng Sun 1 , Zhaohui Wang 4, 5
Affiliation
|
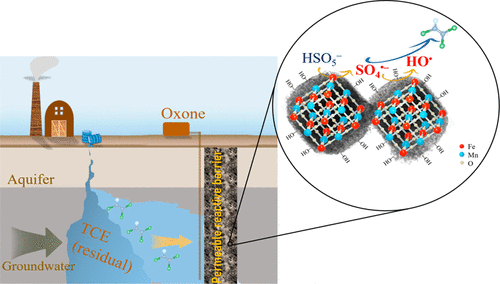
Bimetallic Fe-Mn oxide (BFMO) has been regarded as a promising activator of peroxysulfate (PS), the sustained activity and durability of BFMO for long-term activation of PS in situ, however, is unclear for groundwater remediation. A BFMO (i.e., Mn1.5FeO6.35) was prepared and explored for PS-based in situ chemical oxidation (ISCO) of trichloroethylene (TCE) in sand columns with simulated/actual groundwater (SGW/AGW). The sustained activity of BFMO, oxidant utilization efficiency, and postreaction characterization were particularly investigated. Electron spin resonance (ESR) and radical scavenging tests implied that sulfate radicals (SO4•-) and hydroxyl radicals (HO•) played major roles in degrading TCE, whereas singlet oxygen (1O2) contributed less to TCE degradation by BFMO-activated Oxone. Fast degradation and almost complete dechlorination of TCE in AGW were obtained, with reaction stoichiometry efficiencies (RSE) of ΔTCE/ΔOxone at 3-5%, much higher than those reported RSE values in H2O2-based ISCO (≤0.28%). HCO3- did not show detrimental effect on TCE degradation, and effects of natural organic matters (NOM) were negligible at high Oxone dosage. Postreaction characterizations displayed that the BFMO was remarkably stable with sustained activity for Oxone activation after 115 days of continuous-flow test, which therefore can be promising catalyst for Oxone-based ISCO for TCE-contaminated groundwater remediation.
中文翻译:

用于地下水中三氯乙烯原位化学氧化(ISCO)的双金属Fe-Mn氧化物活化的丙酮:效率,持续活性和机理研究。
双金属Fe-Mn氧化物(BFMO)被认为是有希望的过氧硫酸盐(PS)活化剂,BFMO对于PS原位长期活化的持续活性和耐久性,但是对于地下水修复尚不清楚。制备了BFMO(即Mn1.5FeO6.35),并在模拟/实际地下水(SGW / AGW)的砂柱中研究了三氯乙烯(TCE)的基于PS的原位化学氧化(ISCO)。BFMO的持续活性,氧化剂利用效率和反应后表征进行了专门研究。电子自旋共振(ESR)和自由基清除测试表明,硫酸根(SO4•-)和羟基自由基(HO•)在降解TCE中起主要作用,而单线态氧(1O2)对BFMO活化的Oxone降解TCE的贡献较小。在AGW中,TCE可以快速降解并几乎完全脱氯,而ΔTCE/ΔOxone的反应化学计量效率(RSE)为3-5%,远高于基于H2O2的ISCO中报告的RSE值(≤0.28%)。HCO 3-对TCE的降解没有有害作用,并且在高Oxone剂量下,天然有机物(NOM)的影响可以忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。在高Oxone剂量下,天然有机物(NOM)的影响可忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。在高Oxone剂量下,天然有机物(NOM)的影响可忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。
更新日期:2020-02-28
中文翻译:

用于地下水中三氯乙烯原位化学氧化(ISCO)的双金属Fe-Mn氧化物活化的丙酮:效率,持续活性和机理研究。
双金属Fe-Mn氧化物(BFMO)被认为是有希望的过氧硫酸盐(PS)活化剂,BFMO对于PS原位长期活化的持续活性和耐久性,但是对于地下水修复尚不清楚。制备了BFMO(即Mn1.5FeO6.35),并在模拟/实际地下水(SGW / AGW)的砂柱中研究了三氯乙烯(TCE)的基于PS的原位化学氧化(ISCO)。BFMO的持续活性,氧化剂利用效率和反应后表征进行了专门研究。电子自旋共振(ESR)和自由基清除测试表明,硫酸根(SO4•-)和羟基自由基(HO•)在降解TCE中起主要作用,而单线态氧(1O2)对BFMO活化的Oxone降解TCE的贡献较小。在AGW中,TCE可以快速降解并几乎完全脱氯,而ΔTCE/ΔOxone的反应化学计量效率(RSE)为3-5%,远高于基于H2O2的ISCO中报告的RSE值(≤0.28%)。HCO 3-对TCE的降解没有有害作用,并且在高Oxone剂量下,天然有机物(NOM)的影响可以忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。在高Oxone剂量下,天然有机物(NOM)的影响可忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。在高Oxone剂量下,天然有机物(NOM)的影响可忽略不计。反应后表征表明,BFMO在连续流测试115天后具有明显的稳定性,并具有对Oxone活化的持续活性,因此对于基于Tx污染的地下水的基于Oxone的ISCO而言,有望成为有希望的催化剂。































 京公网安备 11010802027423号
京公网安备 11010802027423号