当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
High-Entropy Alloys as Catalysts for the CO2 and CO Reduction Reactions: Experimental Realization
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acscatal.9b04302 Subramanian Nellaiappan 1 , Nirmal Kumar Katiyar 2 , Ritesh Kumar 3 , Arko Parui 3 , Kirtiman Deo Malviya 4 , K. G. Pradeep 5 , Abhishek K. Singh 3 , Sudhanshu Sharma 1 , Chandra Sekhar Tiwary 6 , Krishanu Biswas 2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-04 , DOI: 10.1021/acscatal.9b04302 Subramanian Nellaiappan 1 , Nirmal Kumar Katiyar 2 , Ritesh Kumar 3 , Arko Parui 3 , Kirtiman Deo Malviya 4 , K. G. Pradeep 5 , Abhishek K. Singh 3 , Sudhanshu Sharma 1 , Chandra Sekhar Tiwary 6 , Krishanu Biswas 2
Affiliation
|
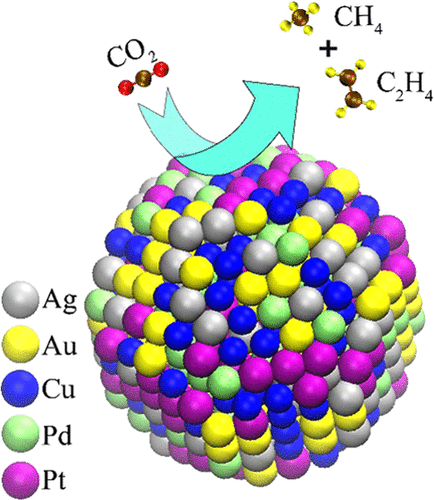
Conversion of carbon dioxide into selective hydrocarbon using a stable catalyst remains a holy grail in the catalysis community. The high overpotential, stability, and selectivity in the use of a single-metal-based catalyst still remain a challenge. In current work, instead of using pure noble metals (Ag, Au, and Pt) as the catalyst, a nanocrystalline high-entropy alloy (HEA: AuAgPtPdCu) has been used for the conversion of CO2 into gaseous hydrocarbons. Utilizing an approach of multimetallic HEA, a faradic efficiency of about 100% toward gaseous products is obtained at a low applied potential (−0.3 V vs reversible hydrogen electrode). The reason behind the catalytic activity and selectivity of the high-entropy alloy (HEA) toward CO2 electroreduction was established through first-principles-based density functional theory (DFT) by comparing it with the pristine Cu(111) surface. This is attributed to the reversal in adsorption trends for two out of the total eight intermediates—*OCH3 and *O on Cu(111) and HEA surfaces.
中文翻译:

高熵合金作为CO 2和CO还原反应的催化剂:实验实现
使用稳定的催化剂将二氧化碳转化为选择性烃仍然是催化领域的圣杯。使用单金属基催化剂的高过电势,稳定性和选择性仍然是一个挑战。在当前的工作中,代替使用纯贵金属(Ag,Au和Pt)作为催化剂,纳米晶高熵合金(HEA:AuAgPtPdCu)已用于将CO 2转化为气态烃。利用多金属HEA的方法,在较低的施加电势下(相对于可逆氢电极为-0.3 V),对气态产物的法拉第效率达到了约100%。高熵合金(HEA)对CO 2的催化活性和选择性背后的原因电还原是通过基于第一原理的密度泛函理论(DFT)与原始Cu(111)表面进行比较而建立的。这归因于全部八个中间体中的两个吸附趋势的逆转-Cu (111)和HEA表面上的* OCH 3和* O。
更新日期:2020-03-05
中文翻译:

高熵合金作为CO 2和CO还原反应的催化剂:实验实现
使用稳定的催化剂将二氧化碳转化为选择性烃仍然是催化领域的圣杯。使用单金属基催化剂的高过电势,稳定性和选择性仍然是一个挑战。在当前的工作中,代替使用纯贵金属(Ag,Au和Pt)作为催化剂,纳米晶高熵合金(HEA:AuAgPtPdCu)已用于将CO 2转化为气态烃。利用多金属HEA的方法,在较低的施加电势下(相对于可逆氢电极为-0.3 V),对气态产物的法拉第效率达到了约100%。高熵合金(HEA)对CO 2的催化活性和选择性背后的原因电还原是通过基于第一原理的密度泛函理论(DFT)与原始Cu(111)表面进行比较而建立的。这归因于全部八个中间体中的两个吸附趋势的逆转-Cu (111)和HEA表面上的* OCH 3和* O。

































 京公网安备 11010802027423号
京公网安备 11010802027423号