当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Higher-Valent Nickel Oxides with Improved Oxygen Evolution Activity and Stability in Alkaline Media Prepared by High-Temperature Treatment of Ni(OH)2
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acscatal.9b04788 Matthias Steimecke 1 , Gerda Seiffarth 1 , Christian Schneemann 1 , Florian Oehler 2 , Stefan Förster 3 , Michael Bron 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acscatal.9b04788 Matthias Steimecke 1 , Gerda Seiffarth 1 , Christian Schneemann 1 , Florian Oehler 2 , Stefan Förster 3 , Michael Bron 1
Affiliation
|
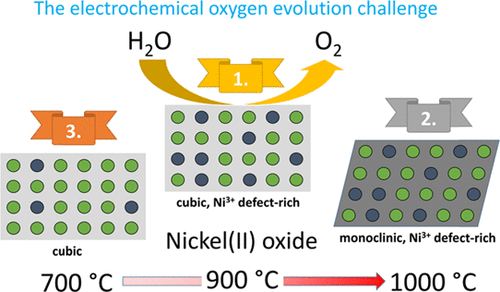
Nickel(II) hydroxide is a well-known material for the oxygen evolution reaction (OER) in alkaline media, particularly when iron is incorporated into its lattice. Moderate heat treatment of nickel(II) hydroxide (≤700 °C) leads to the formation of nickel(II) oxide (nano)particles, which exhibit reduced OER activity the higher the heat treatment temperature was. In this work, we report that heat treatment of nickel(II) hydroxide in air at even higher temperatures (60 min at 900 °C) results in an oxide material with high OER activity superior to that of the nickel(II) hydroxide. Similarly, the stability of the nickel(II) oxide under electrochemical conditions is increased compared to nickel(II) hydroxide. Electrochemical in situ Raman measurements show the formation of surface nickel oxy-hydroxides (NiOOH) at positive potentials and are significantly affected by the initial heat treatment. From XPS, Raman, and XRD results, it is concluded that a Ni3+-enriched phase, possibly a higher-valent mixed nickel oxide, is present at the surface of the nickel(II) oxide sample treated at 900 °C resulting in an increased OER activity compared to NiOOH. This basic understanding of high-temperature-treated nickel oxide may contribute to resolving the present stability issues of OER electrocatalysts and may help to leverage alkaline electrolysis as important key technology for a renewable energy supply.
中文翻译:

高温处理Ni(OH)2制备的碱性介质中具有更高的析氧活性和稳定性的更高价的氧化镍
氢氧化镍(II)是一种在碱性介质中用于氧释放反应(OER)的众所周知的材料,特别是当铁混入其晶格中时。氢氧化镍(II)的适度热处理(≤700°C)导致形成氧化镍(II)(纳米)颗粒,热处理温度越高,其OER活性越低。在这项工作中,我们报告说,即使在更高的温度(900°C下60分钟)中在空气中对氢氧化镍(II)进行热处理,所得到的氧化物材料的OER活性也优于氢氧化镍(II)。类似地,与氢氧化镍(II)相比,在电化学条件下氧化镍(II)的稳定性增加。电化学原位拉曼测量显示在正电势下会形成表面羟基氧化镍(NiOOH),并受到初始热处理的显着影响。根据XPS,拉曼和XRD结果,可以得出结论:在900°C下处理的氧化镍(II)样品表面上存在3+富集相,可能是较高价的混合氧化镍,与NiOOH相比,其OER活性增强。对高温处理的氧化镍的基本了解可能有助于解决OER电催化剂目前的稳定性问题,并可能有助于利用碱性电解作为可再生能源供应的重要关键技术。
更新日期:2020-03-03
中文翻译:

高温处理Ni(OH)2制备的碱性介质中具有更高的析氧活性和稳定性的更高价的氧化镍
氢氧化镍(II)是一种在碱性介质中用于氧释放反应(OER)的众所周知的材料,特别是当铁混入其晶格中时。氢氧化镍(II)的适度热处理(≤700°C)导致形成氧化镍(II)(纳米)颗粒,热处理温度越高,其OER活性越低。在这项工作中,我们报告说,即使在更高的温度(900°C下60分钟)中在空气中对氢氧化镍(II)进行热处理,所得到的氧化物材料的OER活性也优于氢氧化镍(II)。类似地,与氢氧化镍(II)相比,在电化学条件下氧化镍(II)的稳定性增加。电化学原位拉曼测量显示在正电势下会形成表面羟基氧化镍(NiOOH),并受到初始热处理的显着影响。根据XPS,拉曼和XRD结果,可以得出结论:在900°C下处理的氧化镍(II)样品表面上存在3+富集相,可能是较高价的混合氧化镍,与NiOOH相比,其OER活性增强。对高温处理的氧化镍的基本了解可能有助于解决OER电催化剂目前的稳定性问题,并可能有助于利用碱性电解作为可再生能源供应的重要关键技术。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号