Nature ( IF 50.5 ) Pub Date : 2020-02-19 , DOI: 10.1038/s41586-020-2026-1 Brian W Chow 1 , Vicente Nuñez 1 , Luke Kaplan 1 , Adam J Granger 1, 2 , Karina Bistrong 1 , Hannah L Zucker 1 , Payal Kumar 1 , Bernardo L Sabatini 1, 2 , Chenghua Gu 1
|
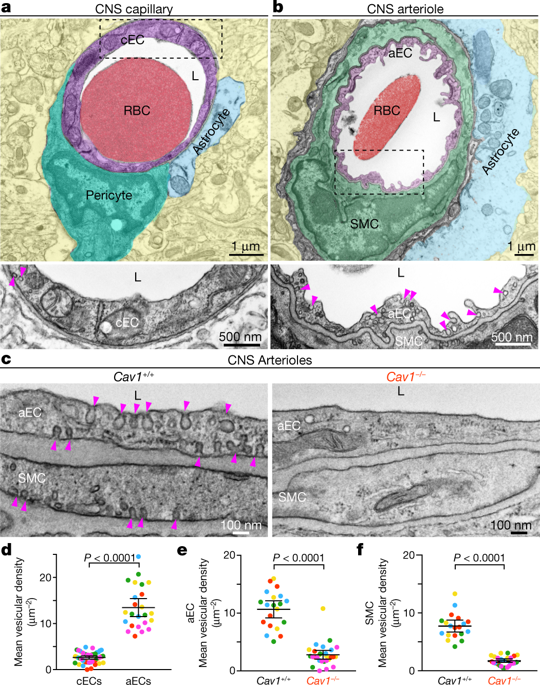
Proper brain function depends on neurovascular coupling: neural activity rapidly increases local blood flow to meet moment-to-moment changes in regional brain energy demand1. Neurovascular coupling is the basis for functional brain imaging2, and impaired neurovascular coupling is implicated in neurodegeneration1. The underlying molecular and cellular mechanisms of neurovascular coupling remain poorly understood. The conventional view is that neurons or astrocytes release vasodilatory factors that act directly on smooth muscle cells (SMCs) to induce arterial dilation and increase local blood flow1. Here, using two-photon microscopy to image neural activity and vascular dynamics simultaneously in the barrel cortex of awake mice under whisker stimulation, we found that arteriolar endothelial cells (aECs) have an active role in mediating neurovascular coupling. We found that aECs, unlike other vascular segments of endothelial cells in the central nervous system, have abundant caveolae. Acute genetic perturbations that eliminated caveolae in aECs, but not in neighbouring SMCs, impaired neurovascular coupling. Notably, caveolae function in aECs is independent of the endothelial NO synthase (eNOS)-mediated NO pathway. Ablation of both caveolae and eNOS completely abolished neurovascular coupling, whereas the single mutants exhibited partial impairment, revealing that the caveolae-mediated pathway in aECs is a major contributor to neurovascular coupling. Our findings indicate that vasodilation is largely mediated by endothelial cells that actively relay signals from the central nervous system to SMCs via a caveolae-dependent pathway.
中文翻译:

中枢神经系统小动脉中的小窝介导神经血管耦合
适当的脑功能取决于神经血管耦合:神经活动迅速增加局部血流量,以满足区域脑能量需求的时刻变化1。神经血管耦合是功能性脑成像2的基础,神经血管耦合受损与神经退行性变1有关。神经血管耦合的潜在分子和细胞机制仍然知之甚少。传统观点认为,神经元或星形胶质细胞释放血管舒张因子,直接作用于平滑肌细胞 (SMC) 以诱导动脉扩张并增加局部血流量1. 在这里,我们使用双光子显微镜在胡须刺激下同时对清醒小鼠桶状皮层的神经活动和血管动力学进行成像,我们发现小动脉内皮细胞 (aEC) 在介导神经血管耦合方面具有积极作用。我们发现,与中枢神经系统内皮细胞的其他血管段不同,aECs 具有丰富的小窝。急性遗传扰动消除了 aECs 中的小窝,但在邻近的 SMCs 中没有,损害了神经血管耦合。值得注意的是,aECs 中的小窝细胞功能不依赖于内皮 NO 合酶 (eNOS) 介导的 NO 途径。Caveolae 和 eNOS 的消融完全消除了神经血管耦合,而单个突变体表现出部分损伤,揭示了aECs中caveolae介导的途径是神经血管耦合的主要贡献者。我们的研究结果表明,血管舒张主要由内皮细胞介导,内皮细胞通过小窝依赖性途径将信号从中枢神经系统主动传递到 SMC。































 京公网安备 11010802027423号
京公网安备 11010802027423号