Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Phosphine-Catalyzed Novel Rearrangement of Vinylcyclopropylketone to Cycloheptenone: A DFT Study.
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b03902 Yong Wu 1 , Mingzhen Li 1 , Lu Jin 2 , Xiang Zhao 1
ACS Omega ( IF 3.7 ) Pub Date : 2020-02-06 , DOI: 10.1021/acsomega.9b03902 Yong Wu 1 , Mingzhen Li 1 , Lu Jin 2 , Xiang Zhao 1
Affiliation
|
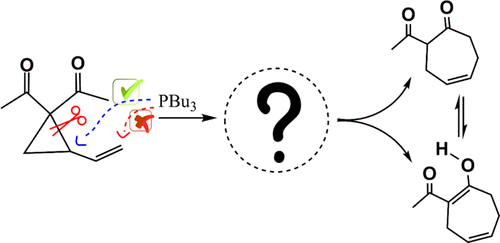
The title reaction is theoretically investigated in detail using density functional theory. Three possible routes starting from keto- or enol-type vinylcyclopropylketone are considered in this work. Results indicate that phosphine catalyst would first attack at the three-membered ring (C3 position) rather than the terminal of alkene (C1 position) in vinylcyclopropylketone. It is found that the two-stage mechanism would be responsible for the title reaction. The first stage is the SN2-type ring-opening of the keto-type vinylcyclopropylketone with phosphine catalyst. After the proton-transfer tautomerisms in the zwitterionic intermediates, the second stage is associated with the 7-endo-trig SN2'-type ring closure of keto- or enol-type zwitterions to furnish seven-membered cyclic products and recover the catalyst. Moreover, it turns out that 7-endo-trig SN2'-type ring closure would be highly asynchronous and could be well described as an addition/elimination process where the ring closure already finishes before the cleavage of the C-P bond. Computational results provide a deep insight into experimental observations.
中文翻译:

膦催化的乙烯基环丙基酮向环庚烯酮的新型重排的机理:DFT研究。
理论上使用密度泛函理论详细研究了标题反应。在这项工作中,考虑了从酮型或烯醇型乙烯基环丙基酮开始的三种可能的途径。结果表明,膦催化剂首先会攻击乙烯基环丙基酮中的三元环(C3位置),而不是烯烃的末端(C1位置)。发现标题机制是由两阶段机制引起的。第一步是用膦催化剂将酮型乙烯基环丙基酮的SN2型开环。在两性离子中间体中发生质子转移互变异构之后,第二阶段与酮或烯醇型两性离子的7-内-trig SN2'型环闭合相关,以提供七元环状产物并回收催化剂。此外,事实证明,7-内-trig SN2'型环闭合是高度异步的,并且可以很好地描述为添加/消除过程,其中环闭合在CP键断裂之前已经完成。计算结果为实验观察提供了深刻的见识。
更新日期:2020-02-18
中文翻译:

膦催化的乙烯基环丙基酮向环庚烯酮的新型重排的机理:DFT研究。
理论上使用密度泛函理论详细研究了标题反应。在这项工作中,考虑了从酮型或烯醇型乙烯基环丙基酮开始的三种可能的途径。结果表明,膦催化剂首先会攻击乙烯基环丙基酮中的三元环(C3位置),而不是烯烃的末端(C1位置)。发现标题机制是由两阶段机制引起的。第一步是用膦催化剂将酮型乙烯基环丙基酮的SN2型开环。在两性离子中间体中发生质子转移互变异构之后,第二阶段与酮或烯醇型两性离子的7-内-trig SN2'型环闭合相关,以提供七元环状产物并回收催化剂。此外,事实证明,7-内-trig SN2'型环闭合是高度异步的,并且可以很好地描述为添加/消除过程,其中环闭合在CP键断裂之前已经完成。计算结果为实验观察提供了深刻的见识。






























 京公网安备 11010802027423号
京公网安备 11010802027423号