当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Role of Inorganic and Organic Interfaces on CO2 and CH4 Partitioning: Case Study of Silica, Illite, Calcite, and Kerogen Nanopores on Gas Adsorption and Nanoscale Transport Behaviors
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.energyfuels.0c00052 Sohaib Mohammed 1 , Greeshma Gadikota 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.energyfuels.0c00052 Sohaib Mohammed 1 , Greeshma Gadikota 1
Affiliation
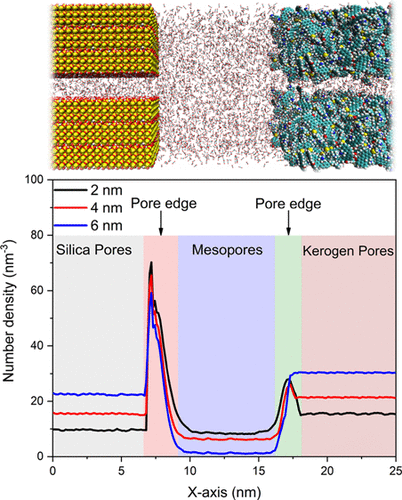
|
The adsorption, partitioning, and diffusion of CO2 and CH4 in organic–inorganic pores composed of kerogen with silica, illite, or calcite are studied using density functional theory and classical molecular dynamics simulations. The adsorption and partitioning behavior of CO2 and CH4 molecules is found to be a function of chemistry of the solid interface, pore size, and surface area. CO2 molecules are preferentially adsorbed compared to CH4 molecules on silica, illite, calcite, and kerogen surfaces due to significant contributions of electrostatic interactions with the atoms of the associated surfaces. CO2 and CH4 molecules display higher adsorption energy on calcite and silica compared to those on illite and kerogen because of enhanced interactions with the positively charged calcium ions on the calcite surface and the hydroxyl group (−OH) on the silica surface. Enhanced internal nanopores and the availability of adsorption active sites in illite and kerogen matrices aid in higher partitioning of the gas molecules into these pores. CO2 has a significant influence on the clustering and swelling of kerogen fragments compared to CH4. Higher CO2 adsorption on inorganic and kerogen surfaces results in lower overall self-diffusivities compared to CH4. This study illustrates surface and pore size controls on the adsorption, partitioning, and self-diffusivities of CO2 and CH4 at conditions relevant for subsurface energy applications. The chemistry of the surfaces along with the availability of internal pores and active adsorption sites influences the adsorption behavior of gases. Our results suggest that CO2 stored in depleted gas reservoirs may preferentially inhabit nanoconfined pores.
中文翻译:

探索无机和有机界面在CO 2和CH 4分配中的作用:二氧化硅,伊利石,方解石和干酪根纳米孔对气体吸附和纳米尺度迁移行为的案例研究
利用密度泛函理论和经典的分子动力学模拟研究了干酪根与二氧化硅,伊利石或方解石组成的有机-无机孔隙中CO 2和CH 4的吸附,分配和扩散。发现CO 2和CH 4分子的吸附和分配行为是固体界面化学性质,孔径和表面积的函数。与CH 4分子相比,由于与相关表面原子的静电相互作用的显着贡献,与CH 4分子相比,CO 2分子优先吸附在二氧化硅,伊利石,方解石和干酪根表面。CO 2和CH 4与方解石和干酪根上的分子相比,方解石和硅石上的分子具有更高的吸附能,这是因为与方解石表面的带正电的钙离子和二氧化硅表面的羟基(-OH)相互作用增强。增强的内部纳米孔以及伊利石和干酪根基质中吸附活性位点的可用性有助于气体分子在这些孔中的分配更高。与CH 4相比,CO 2对干酪根碎片的聚集和膨胀具有显着影响。与CH 4相比,较高的CO 2在无机和干酪根表面上的吸附导致较低的整体自扩散性。这项研究说明了在与地下能量应用相关的条件下,表面和孔径对CO 2和CH 4的吸附,分配和自扩散的控制。表面的化学性质以及内部孔隙和活性吸附位的可用性会影响气体的吸附行为。我们的结果表明,储存在贫气气藏中的CO 2可能优先居住在纳米约束的孔隙中。
更新日期:2020-02-28
中文翻译:

探索无机和有机界面在CO 2和CH 4分配中的作用:二氧化硅,伊利石,方解石和干酪根纳米孔对气体吸附和纳米尺度迁移行为的案例研究
利用密度泛函理论和经典的分子动力学模拟研究了干酪根与二氧化硅,伊利石或方解石组成的有机-无机孔隙中CO 2和CH 4的吸附,分配和扩散。发现CO 2和CH 4分子的吸附和分配行为是固体界面化学性质,孔径和表面积的函数。与CH 4分子相比,由于与相关表面原子的静电相互作用的显着贡献,与CH 4分子相比,CO 2分子优先吸附在二氧化硅,伊利石,方解石和干酪根表面。CO 2和CH 4与方解石和干酪根上的分子相比,方解石和硅石上的分子具有更高的吸附能,这是因为与方解石表面的带正电的钙离子和二氧化硅表面的羟基(-OH)相互作用增强。增强的内部纳米孔以及伊利石和干酪根基质中吸附活性位点的可用性有助于气体分子在这些孔中的分配更高。与CH 4相比,CO 2对干酪根碎片的聚集和膨胀具有显着影响。与CH 4相比,较高的CO 2在无机和干酪根表面上的吸附导致较低的整体自扩散性。这项研究说明了在与地下能量应用相关的条件下,表面和孔径对CO 2和CH 4的吸附,分配和自扩散的控制。表面的化学性质以及内部孔隙和活性吸附位的可用性会影响气体的吸附行为。我们的结果表明,储存在贫气气藏中的CO 2可能优先居住在纳米约束的孔隙中。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号