Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Coupling Chlorin e6 to the surface of Nanoscale Gas Vesicles strongly enhance their intracellular delivery and photodynamic killing of cancer cells.
Scientific Reports ( IF 3.8 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41598-020-59584-1 Ann Fernando 1, 2 , Jean Gariépy 1, 2, 3
Scientific Reports ( IF 3.8 ) Pub Date : 2020-02-18 , DOI: 10.1038/s41598-020-59584-1 Ann Fernando 1, 2 , Jean Gariépy 1, 2, 3
Affiliation
|
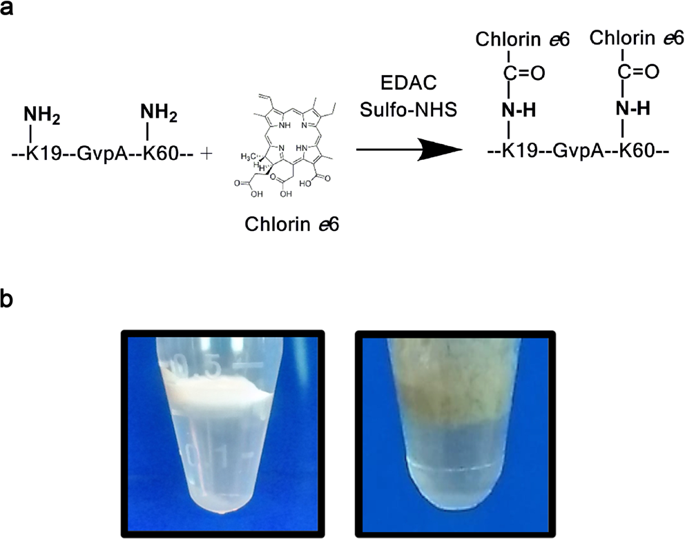
Protein-based nanobubbles such as halophilic archaeabacterial gas vesicles (GVs) represent a new class of stable, homogeneous nanoparticles with acoustic properties that allow them to be visualized by ultrasound (US) waves. To design GVs as theranostic agents, we modified them to respond to light, with a view to locally generate reactive oxygen species that can kill cancer cells. Specifically, up to 60,000 photoreactive chlorin e6 (Ce6) molecules were chemically attached to lysine ε-amino groups present on the surface of each purified Halobacterium sp. NRC-1 GV. The resulting fluorescent NRC-1 Ce6-GVs have dimensions comparable to that of native GVs and were efficiently taken up by human breast [MCF-7] and human hypopharyngeal [FaDu-GFP] cancer cells as monitored by confocal microscopy and flow cytometry. When exposed to light, internalized Ce6-GVs were 200-fold more effective on a molar basis than free Ce6 at killing cells. These results demonstrate the potential of Ce6-GVs as novel and promising nanomaterials for image-guided photodynamic therapy.
中文翻译:

将氯绿素e6偶联至纳米级气体囊泡的表面可大大增强其细胞内递送和对癌细胞的光动力杀伤。
基于蛋白质的纳米气泡(例如,嗜盐古细菌气体囊泡(GV))代表了一类新型稳定,均质的纳米粒子,其纳米粒子具有声学特性,可通过超声波(US)进行可视化。为了将GV设计为治疗试剂,我们修改了它们以响应光,以局部产生可杀死癌细胞的活性氧。具体而言,将多达60,000个光反应性二氢卟酚e6(Ce6)分子化学连接到每个纯化Halobacterium sp。表面上存在的赖氨酸ε-氨基。NRC-1 GV。所得荧光NRC-1 Ce6-GV具有与天然GV相当的尺寸,并且通过共聚焦显微镜和流式细胞术监测,可被人乳腺[MCF-7]和人咽部[FaDu-GFP]癌细胞有效吸收。当暴露在光线下时 在杀死细胞时,内化的Ce6-GV的摩尔效率比游离Ce6高200倍。这些结果证明了Ce6-GVs作为用于图像引导的光动力疗法的新型和有前途的纳米材料的潜力。
更新日期:2020-02-18
中文翻译:

将氯绿素e6偶联至纳米级气体囊泡的表面可大大增强其细胞内递送和对癌细胞的光动力杀伤。
基于蛋白质的纳米气泡(例如,嗜盐古细菌气体囊泡(GV))代表了一类新型稳定,均质的纳米粒子,其纳米粒子具有声学特性,可通过超声波(US)进行可视化。为了将GV设计为治疗试剂,我们修改了它们以响应光,以局部产生可杀死癌细胞的活性氧。具体而言,将多达60,000个光反应性二氢卟酚e6(Ce6)分子化学连接到每个纯化Halobacterium sp。表面上存在的赖氨酸ε-氨基。NRC-1 GV。所得荧光NRC-1 Ce6-GV具有与天然GV相当的尺寸,并且通过共聚焦显微镜和流式细胞术监测,可被人乳腺[MCF-7]和人咽部[FaDu-GFP]癌细胞有效吸收。当暴露在光线下时 在杀死细胞时,内化的Ce6-GV的摩尔效率比游离Ce6高200倍。这些结果证明了Ce6-GVs作为用于图像引导的光动力疗法的新型和有前途的纳米材料的潜力。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号