当前位置:
X-MOL 学术
›
Cancer Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of anlotinib as a third- or further-line therapy in advanced non-small cell lung cancer patients with different histologic types: Subgroup analysis in the ALTER0303 trial.
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-02-16 , DOI: 10.1002/cam4.2913 Ying Cheng 1 , Baohui Han 2 , Kai Li 3 , Qiming Wang 4 , Li Zhang 5 , Jianhua Shi 6 , Zhehai Wang 7 , Jianxing He 8 , Yuankai Shi 9 , Weiqiang Chen 10 , Xiuwen Wang 11 , Yi Luo 12 , Kejun Nan 13 , Faguang Jin 14 , Baolan Li 15 , Jing Zhu 16
Cancer Medicine ( IF 2.9 ) Pub Date : 2020-02-16 , DOI: 10.1002/cam4.2913 Ying Cheng 1 , Baohui Han 2 , Kai Li 3 , Qiming Wang 4 , Li Zhang 5 , Jianhua Shi 6 , Zhehai Wang 7 , Jianxing He 8 , Yuankai Shi 9 , Weiqiang Chen 10 , Xiuwen Wang 11 , Yi Luo 12 , Kejun Nan 13 , Faguang Jin 14 , Baolan Li 15 , Jing Zhu 16
Affiliation
|
BACKGROUND
Anlotinib showed significant survival benefits in advanced non-small cell lung cancer (NSCLC) patients as a third- or further-line treatment in the ALTER0303 trial. We aimed to evaluate the efficacy of anlotinib in patients with different histologies.
METHODS
The ALTER0303 trial was a randomized, open-label, phase 3 study of anlotinib in NSCLC patients previously treated with at least two lines of chemotherapy or a tyrosine kinase inhibitor (TKI) in 31 centers in China. Patients were randomly assigned at a 2:1 ratio to receive anlotinib (12 mg QD from days 1 to 14 of a 21-day cycle) or placebo until progression or intolerable toxicity. The primary endpoint was overall survival (OS). We assessed the efficacy of anlotinib in histological subgroups in the full analysis set.
RESULTS
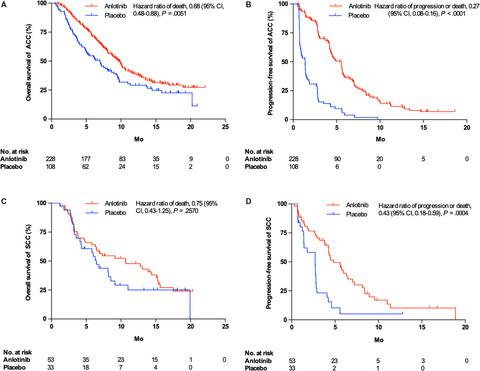
In the ALTER0303 trial, 336 patients had the histological subtype of adenocarcinoma (ACC), 86 patients had the histological subtype of squamous cell carcinoma (SCC), and 15 patients had another subtype. In the ACC subgroup, the median OS time was significantly improved with anlotinib compared with placebo (9.6 months vs 6.9 months, P = .0051), as was the median progression-free survival (PFS) time (5.5 months vs 1.4 months, P < .0001). In the SCC subgroup, the median OS time was 10.7 months in the anlotinib group and 6.5 months in the placebo group (P = .2570), and the median PFS time was 4.8 months and 2.7 months (P = .0004), respectively. The common adverse events observed in the SCC and ACC subgroups were similar.
CONCLUSIONS
Our findings suggest that anlotinib significantly improves PFS and OS in ACC patients and has a tendency to prolong survival in SCC patients.
更新日期:2020-02-16






























 京公网安备 11010802027423号
京公网安备 11010802027423号