当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Highly Soluble 1,4-Diaminoanthraquinone Derivative for Nonaqueous Symmetric Redox Flow Batteries
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-02-26 , DOI: 10.1021/acssuschemeng.9b07244
Pieter Geysens 1 , Yun Li 2 , Ivo Vankelecom 2 , Jan Fransaer 3 , Koen Binnemans 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2020-02-26 , DOI: 10.1021/acssuschemeng.9b07244
Pieter Geysens 1 , Yun Li 2 , Ivo Vankelecom 2 , Jan Fransaer 3 , Koen Binnemans 1
Affiliation
|
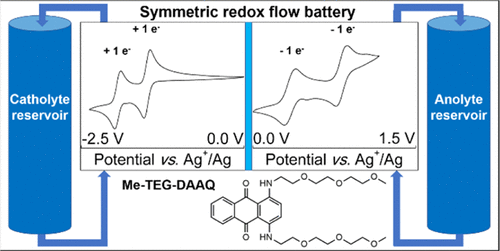
Nonaqueous redox flow batteries (RFBs) based on redox-active organic molecules are regarded as a promising technology for large-scale grid energy storage. 1,4-Diaminoanthraquinones (DAAQs) are particularly interesting as active species because they can have up to five different electrochemically accessible oxidation states, but their practical usability is limited because of their low solubility in commonly used polar organic solvents. We present a DAAQ derivative, 1,4-bis((2-(2-(2-methoxyethoxy)ethoxy)ethyl)amino)anthracene-9,10-dione (Me-TEG-DAAQ), which can be synthesized from inexpensive precursors and overcomes this disadvantage of low solubility. This derivative has a low melting point (25 °C), a concentration exceeding 2.2 mol L–1 in the pure state, and is miscible in any ratio with polar organic solvents such as acetonitrile (MeCN) and 1,2-dimethoxyethane. Cyclic voltammetry experiments show that two anodic and two cathodic one-electron redox transitions are electrochemically accessible and highly reversible, with an interval of 1.8 V between the two inner redox couples and 2.7 V between the two outer redox couples. Proof-of-concept galvanostatic cycling experiments were conducted on dilute solutions of Me-TEG-DAAQ in a simple symmetric electrochemical cell. When only the two inner redox couples are considered, the electrochemical cell can achieve specific capacities close to the theoretical value (2.68 A h L–1), with only limited capacity fading (i.e., <20%) over 100 cycles. When the two outer redox couples are considered as well, the cell can achieve its theoretical capacity (5.36 A h L–1), but faster capacity fading occurs. This combination of high reversibility, high theoretical cell potential (2.7 V), high theoretical energy density (>49 W h L–1), and limited capacity fading in an electrochemical cell based on one single active species is unprecedented in the RFB literature.
中文翻译:

非水对称氧化还原液流电池的高可溶性1,4-二氨基蒽醌衍生物
基于氧化还原活性有机分子的非水氧化还原液流电池(RFB)被认为是用于大规模电网储能的有前途的技术。1,4-二氨基蒽醌(DAAQs)作为活性物质特别有趣,因为它们可以具有多达五个不同的电化学可及的氧化态,但是由于它们在常用的极性有机溶剂中的溶解度低,因此其实用性受到限制。我们提出了一种DAAQ衍生物1,4-双((2-(2-(2-(2-甲氧基乙氧基)乙氧基)乙基)氨基)蒽-9,10-二酮(Me-TEG-DAAQ),可以用廉价的方法合成克服了低溶解度的缺点。该衍生物的熔点低(25°C),浓度超过2.2 mol L –1呈纯净状态,可与极性有机溶剂(如乙腈(MeCN)和1,2-二甲氧基乙烷)以任何比例混溶。循环伏安法实验表明,两个阳极和两个阴极单电子氧化还原跃迁是电化学可接近且高度可逆的,两个内部氧化还原对之间的间隔为1.8 V,两个外部氧化还原对之间的间隔为2.7V。在简单的对称电化学池中,对Me-TEG-DAAQ的稀溶液进行了概念验证的恒电流循环实验。如果仅考虑两个内部氧化还原对,则电化学电池可达到接近理论值的比容量(2.68 A h L –1),在100个周期内仅出现有限的容量衰减(即<20%)。当同时考虑两个外部氧化还原对时,电池可以达到其理论容量(5.36 A h L –1),但容量衰减更快。在RFB文献中,这种高可逆性,高理论电池电势(2.7 V),高理论能量密度(> 49 W h L –1)和有限容量褪色的组合在基于一个单一活性物质的电化学电池中是空前的。
更新日期:2020-02-27
中文翻译:

非水对称氧化还原液流电池的高可溶性1,4-二氨基蒽醌衍生物
基于氧化还原活性有机分子的非水氧化还原液流电池(RFB)被认为是用于大规模电网储能的有前途的技术。1,4-二氨基蒽醌(DAAQs)作为活性物质特别有趣,因为它们可以具有多达五个不同的电化学可及的氧化态,但是由于它们在常用的极性有机溶剂中的溶解度低,因此其实用性受到限制。我们提出了一种DAAQ衍生物1,4-双((2-(2-(2-(2-甲氧基乙氧基)乙氧基)乙基)氨基)蒽-9,10-二酮(Me-TEG-DAAQ),可以用廉价的方法合成克服了低溶解度的缺点。该衍生物的熔点低(25°C),浓度超过2.2 mol L –1呈纯净状态,可与极性有机溶剂(如乙腈(MeCN)和1,2-二甲氧基乙烷)以任何比例混溶。循环伏安法实验表明,两个阳极和两个阴极单电子氧化还原跃迁是电化学可接近且高度可逆的,两个内部氧化还原对之间的间隔为1.8 V,两个外部氧化还原对之间的间隔为2.7V。在简单的对称电化学池中,对Me-TEG-DAAQ的稀溶液进行了概念验证的恒电流循环实验。如果仅考虑两个内部氧化还原对,则电化学电池可达到接近理论值的比容量(2.68 A h L –1),在100个周期内仅出现有限的容量衰减(即<20%)。当同时考虑两个外部氧化还原对时,电池可以达到其理论容量(5.36 A h L –1),但容量衰减更快。在RFB文献中,这种高可逆性,高理论电池电势(2.7 V),高理论能量密度(> 49 W h L –1)和有限容量褪色的组合在基于一个单一活性物质的电化学电池中是空前的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号