当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Application of 3-Bromo-1,2,4,5-Tetrazine for Protein Labeling to Trigger Click-to-Release Biorthogonal Reactions.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00052 Enric Ros 1 , Marina Bellido 1 , Xavier Verdaguer 1, 2 , Lluís Ribas de Pouplana 1, 3 , Antoni Riera 1, 2
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-02-14 , DOI: 10.1021/acs.bioconjchem.0c00052 Enric Ros 1 , Marina Bellido 1 , Xavier Verdaguer 1, 2 , Lluís Ribas de Pouplana 1, 3 , Antoni Riera 1, 2
Affiliation
|
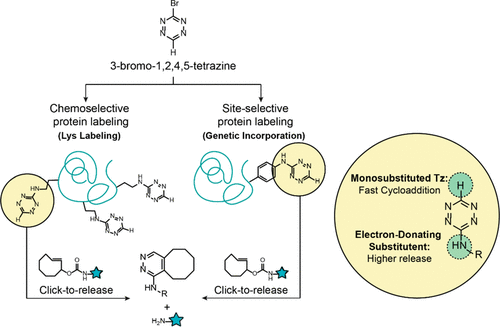
3-Bromo-1,2,4,5-tetrazine has been synthesized in an oxidant- and metal-free method. The synthesis is scalable and relies on inexpensive starting materials. 3-Bromo-1,2,4,5-tetrazine can undergo nucleophilic aromatic substitutions with differently substituted heteroatoms under mild conditions. In particular, its excellent reactivity has been used to attain chemoselective protein labeling. The resulting labeled lysines can react with strained dienophiles to trigger fast click-to-release (CtR) biorthogonal reactions. The characterization of the CtR reaction in physiological conditions and a therapeutically relevant example with the monoclonal antibody Trastuzumab to showcase its application is presented. Finally, 3-bromo-1,2,4,5-tetrazine has been used to achieve site-selective protein labeling through the genetic incorporation of the first unnatural amino acid bearing an unsubstituted 1,2,4,5-tetrazin-3-yl functionality, which can also undergo CtR reactions.
中文翻译:

3-Bromo-1,2,4,5-Tetrazine的合成及其在蛋白质标记中的应用,以触发点击释放双正交反应。
3-溴-1,2,4,5-四嗪已通过无氧化剂和无金属的方法合成。该合成是可扩展的并且依赖于廉价的起始原料。3-溴-1,2,4,5-四嗪可以在温和的条件下通过不同取代的杂原子进行亲核芳香取代。特别地,其优异的反应性已用于获得化学选择性蛋白标记。生成的标记的赖氨酸可以与紧张的亲双烯体反应,从而触发快速点击释放(CtR)生物正交反应。提出了在生理条件下CtR反应的表征,以及使用单克隆抗体曲妥珠单抗的治疗相关实例以展示其应用。最后是3-bromo-1,2,4
更新日期:2020-02-27
中文翻译:

3-Bromo-1,2,4,5-Tetrazine的合成及其在蛋白质标记中的应用,以触发点击释放双正交反应。
3-溴-1,2,4,5-四嗪已通过无氧化剂和无金属的方法合成。该合成是可扩展的并且依赖于廉价的起始原料。3-溴-1,2,4,5-四嗪可以在温和的条件下通过不同取代的杂原子进行亲核芳香取代。特别地,其优异的反应性已用于获得化学选择性蛋白标记。生成的标记的赖氨酸可以与紧张的亲双烯体反应,从而触发快速点击释放(CtR)生物正交反应。提出了在生理条件下CtR反应的表征,以及使用单克隆抗体曲妥珠单抗的治疗相关实例以展示其应用。最后是3-bromo-1,2,4





















































 京公网安备 11010802027423号
京公网安备 11010802027423号