当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A new monooxygenase from Herbaspirillum huttiense catalyzed highly enantioselective epoxidation of allylbenzenes and allylic alcohols
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-02-14 , DOI: 10.1039/d0cy00081g Hui Lin 1, 2, 3 , Yanhong Tang 1, 2, 3 , Shuang Dong 1, 2, 3 , Ruibo Lang 1, 2, 3 , Hongge Chen 1, 2, 3
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-02-14 , DOI: 10.1039/d0cy00081g Hui Lin 1, 2, 3 , Yanhong Tang 1, 2, 3 , Shuang Dong 1, 2, 3 , Ruibo Lang 1, 2, 3 , Hongge Chen 1, 2, 3
Affiliation
|
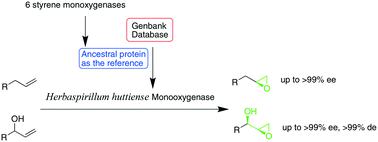
Asymmetric epoxidation is a green route to enantiopure epoxides, but often suffers from low enantioselectivity toward unconjugated terminal alkenes. Mining of the NCBI non-redundant protein sequences with a reconstructed ancestral sequence based on six styrene monooxygenases identified a monooxygenase (HhMo) from Herbaspirillum huttiense with 29.6–32.3% sequence identity with styrene monooxygenases, which was previously annotated as an alanine phosphoribitol ligase. HhMo catalyzed the epoxidation of allylbenzenes with moderate to excellent enantioselectivity yielding the corresponding epoxides in up to 99% ee. The HhMo-catalyzed epoxidation could also achieve the kinetic resolution of racemic secondary allylic alcohols, yielding the corresponding epoxides with up to 50% yields, as well as excellent enantio- and diastereoselectivity (up to >99% ee and >99% de) within 20–60 min, making a greener strategy for the production of valuable enantiopure glycidol derivatives in the fine chemical and pharmaceutical industries.
中文翻译:

来自胡氏螺旋藻的一种新的单加氧酶催化烯丙基苯和烯丙醇的高度对映选择性环氧化
不对称环氧化是制备对映纯环氧化物的绿色途径,但通常对未共轭末端烯烃的对映选择性低。基于六个苯乙烯单加氧酶的祖先序列重构的NCBI非冗余蛋白序列的挖掘,从胡氏螺旋藻中鉴定出单加氧酶(HhMo)与苯乙烯单加氧酶具有29.6–32.3%的序列同一性,以前被标注为丙氨酸磷酸核糖醇连接酶。HhMo以中等至优异的对映选择性催化烯丙基苯的环氧化反应,可产生高达99%ee的相应环氧化物。HhMo催化的环氧化还可以实现外消旋仲烯丙醇的动力学拆分,得到相应的环氧化物,其收率高达50%,并且在其中具有出色的对映和非对映选择性(高达> 99%ee和> 99%de) 20–60分钟,为精细化工和制药业生产有价值的对映纯缩水甘油缩水甘油酯制定了更环保的策略。
更新日期:2020-02-14
中文翻译:

来自胡氏螺旋藻的一种新的单加氧酶催化烯丙基苯和烯丙醇的高度对映选择性环氧化
不对称环氧化是制备对映纯环氧化物的绿色途径,但通常对未共轭末端烯烃的对映选择性低。基于六个苯乙烯单加氧酶的祖先序列重构的NCBI非冗余蛋白序列的挖掘,从胡氏螺旋藻中鉴定出单加氧酶(HhMo)与苯乙烯单加氧酶具有29.6–32.3%的序列同一性,以前被标注为丙氨酸磷酸核糖醇连接酶。HhMo以中等至优异的对映选择性催化烯丙基苯的环氧化反应,可产生高达99%ee的相应环氧化物。HhMo催化的环氧化还可以实现外消旋仲烯丙醇的动力学拆分,得到相应的环氧化物,其收率高达50%,并且在其中具有出色的对映和非对映选择性(高达> 99%ee和> 99%de) 20–60分钟,为精细化工和制药业生产有价值的对映纯缩水甘油缩水甘油酯制定了更环保的策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号