当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-functionalization of dibenzothiophene to functionalized biphenyls via a photoinduced thia-Baeyer-Villiger oxidation.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14522-7 Xiaofeng Ma 1, 2 , Yazhou Liu 2 , Le Du 2, 3 , Jingwei Zhou 4 , István E Markó 2
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-14 , DOI: 10.1038/s41467-020-14522-7 Xiaofeng Ma 1, 2 , Yazhou Liu 2 , Le Du 2, 3 , Jingwei Zhou 4 , István E Markó 2
Affiliation
|
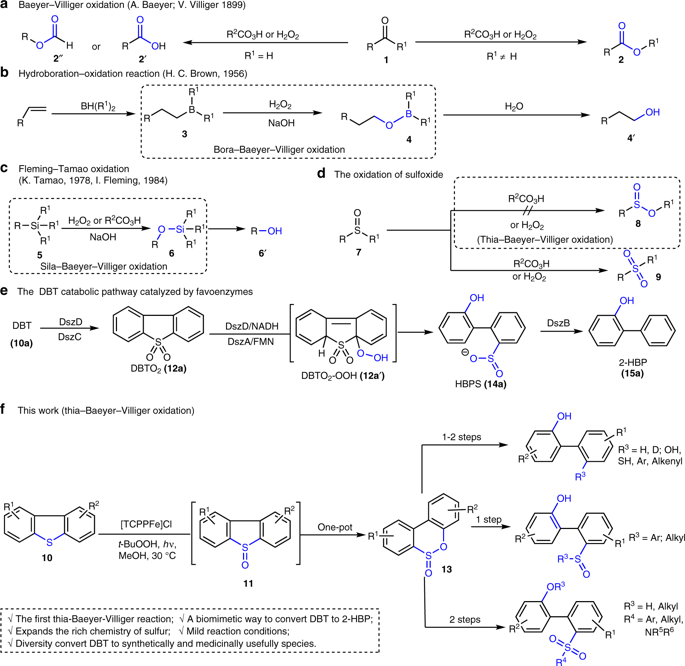
The Baeyer-Villiger reaction is used extensively in organic chemistry. Sila- and bora-variants have also been documented widely, with these processes underpinning, for example, the Fleming-Tamao oxidation and hydroborative alkene hydration, respectively. By contrast, the development of thia-Baeyer-Villiger reactions involving sulfoxides has long been considered unlikely because competitive oxidation to the sulfone occurs exclusively. Here, we disclose a photoinduced thia-Baeyer-Villiger-type oxidations; specifically, we find that exposure of dibenzothiophene (DBT) derivatives to an iron porphyrin catalyst under Ultraviolet irradiation in the presence of t-BuOOH generates sulfinic esters in up to 87% yield. The produced sulfinic esters are transformed to a variety of biphenyl substrates including biphenyl sulfoxides, sulfones and sulfonamides in 1-2 steps. These results provide a mild process for the selective functionalization of sulfur compounds, and offer a biomimetic approach to convert DBT into 2-hydroxybiphenyl under controllable stepwise pathway. Based upon experimental evidences and DFT calculation, a mechanism is proposed.
中文翻译:

通过光诱导的thia-Baeyer-Villiger氧化将二苯并噻吩后官能化为官能化联苯。
Baeyer-Villiger反应被广泛用于有机化学中。Sila和bora变体也得到了广泛的文献报道,这些方法分别支持Fleming-Tamao氧化和硼氢化硼氢化。相比之下,长期以来一直认为不太可能发展涉及亚砜的thia-Baeyer-Villiger反应,因为与砜的竞争性氧化仅会发生。在这里,我们公开了一种光诱导的thia-Baeyer-Villiger型氧化。具体而言,我们发现在t-BuOOH存在下,在紫外线照射下,二苯并噻吩(DBT)衍生物暴露于铁卟啉催化剂会生成亚磺酸酯,产率高达87%。产生的亚磺酸酯被转化为多种联苯底物,包括联苯亚砜,1-2步完成砜和磺酰胺的处理。这些结果为硫化合物的选择性功能化提供了温和的过程,并提供了仿生方法,以可控的逐步途径将DBT转化为2-羟基联苯。基于实验证据和DFT计算,提出了一种机理。
更新日期:2020-02-14
中文翻译:

通过光诱导的thia-Baeyer-Villiger氧化将二苯并噻吩后官能化为官能化联苯。
Baeyer-Villiger反应被广泛用于有机化学中。Sila和bora变体也得到了广泛的文献报道,这些方法分别支持Fleming-Tamao氧化和硼氢化硼氢化。相比之下,长期以来一直认为不太可能发展涉及亚砜的thia-Baeyer-Villiger反应,因为与砜的竞争性氧化仅会发生。在这里,我们公开了一种光诱导的thia-Baeyer-Villiger型氧化。具体而言,我们发现在t-BuOOH存在下,在紫外线照射下,二苯并噻吩(DBT)衍生物暴露于铁卟啉催化剂会生成亚磺酸酯,产率高达87%。产生的亚磺酸酯被转化为多种联苯底物,包括联苯亚砜,1-2步完成砜和磺酰胺的处理。这些结果为硫化合物的选择性功能化提供了温和的过程,并提供了仿生方法,以可控的逐步途径将DBT转化为2-羟基联苯。基于实验证据和DFT计算,提出了一种机理。















































 京公网安备 11010802027423号
京公网安备 11010802027423号