当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cross-talks of glycosylphosphatidylinositol biosynthesis with glycosphingolipid biosynthesis and ER-associated degradation.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41467-020-14678-2 Yicheng Wang 1, 2 , Yusuke Maeda 1 , Yi-Shi Liu 3 , Yoko Takada 2 , Akinori Ninomiya 1 , Tetsuya Hirata 1, 2, 4 , Morihisa Fujita 3 , Yoshiko Murakami 1, 2 , Taroh Kinoshita 1, 2
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41467-020-14678-2 Yicheng Wang 1, 2 , Yusuke Maeda 1 , Yi-Shi Liu 3 , Yoko Takada 2 , Akinori Ninomiya 1 , Tetsuya Hirata 1, 2, 4 , Morihisa Fujita 3 , Yoshiko Murakami 1, 2 , Taroh Kinoshita 1, 2
Affiliation
|
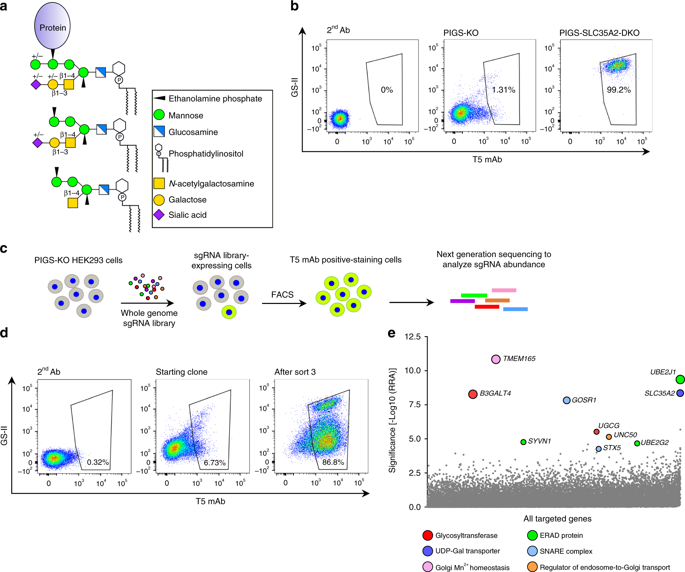
Glycosylphosphatidylinositol (GPI)-anchored proteins and glycosphingolipids interact with each other in the mammalian plasma membranes, forming dynamic microdomains. How their interaction starts in the cells has been unclear. Here, based on a genome-wide CRISPR-Cas9 genetic screen for genes required for GPI side-chain modification by galactose in the Golgi apparatus, we report that β1,3-galactosyltransferase 4 (B3GALT4), the previously characterized GM1 ganglioside synthase, additionally functions in transferring galactose to the N-acetylgalactosamine side-chain of GPI. Furthermore, B3GALT4 requires lactosylceramide for the efficient GPI side-chain galactosylation. Thus, our work demonstrates previously unexpected functional relationships between GPI-anchored proteins and glycosphingolipids in the Golgi. Through the same screening, we also show that GPI biosynthesis in the endoplasmic reticulum (ER) is severely suppressed by ER-associated degradation to prevent GPI accumulation when the transfer of synthesized GPI to proteins is defective. Our data demonstrates cross-talks of GPI biosynthesis with glycosphingolipid biosynthesis and the ER quality control system.
中文翻译:

糖基磷脂酰肌醇生物合成与糖鞘脂生物合成和ER相关降解的串扰。
糖基磷脂酰肌醇(GPI)锚定的蛋白质和糖鞘脂在哺乳动物的质膜中相互作用,形成动态的微区。它们在细胞中的相互作用如何开始尚不清楚。在这里,基于高尔基体中半乳糖修饰GPI侧链所需的基因的全基因组CRISPR-Cas9遗传筛选,我们报道了以前表征为GM1神经节苷脂合酶的β1,3-半乳糖基转移酶4(B3GALT4)。在将半乳糖转移至GPI的N-乙酰半乳糖胺侧链中发挥功能。此外,B3GALT4需要乳糖基神经酰胺才能有效进行GPI侧链半乳糖基化。因此,我们的工作证明了高尔基体中GPI锚定的蛋白与糖鞘脂之间的出乎意料的功能关系。通过相同的筛选 我们还表明内质网(ER)中的GPI生物合成受到ER相关降解的严重抑制,从而在合成GPI向蛋白质的转移有缺陷时防止GPI积累。我们的数据证明了GPI生物合成与糖鞘脂生物合成和ER质量控制系统之间的相互影响。
更新日期:2020-02-13
中文翻译:

糖基磷脂酰肌醇生物合成与糖鞘脂生物合成和ER相关降解的串扰。
糖基磷脂酰肌醇(GPI)锚定的蛋白质和糖鞘脂在哺乳动物的质膜中相互作用,形成动态的微区。它们在细胞中的相互作用如何开始尚不清楚。在这里,基于高尔基体中半乳糖修饰GPI侧链所需的基因的全基因组CRISPR-Cas9遗传筛选,我们报道了以前表征为GM1神经节苷脂合酶的β1,3-半乳糖基转移酶4(B3GALT4)。在将半乳糖转移至GPI的N-乙酰半乳糖胺侧链中发挥功能。此外,B3GALT4需要乳糖基神经酰胺才能有效进行GPI侧链半乳糖基化。因此,我们的工作证明了高尔基体中GPI锚定的蛋白与糖鞘脂之间的出乎意料的功能关系。通过相同的筛选 我们还表明内质网(ER)中的GPI生物合成受到ER相关降解的严重抑制,从而在合成GPI向蛋白质的转移有缺陷时防止GPI积累。我们的数据证明了GPI生物合成与糖鞘脂生物合成和ER质量控制系统之间的相互影响。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号