当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Siroheme synthase orients substrates for dehydrogenase and chelatase activities in a common active site.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41467-020-14722-1 Joseph M Pennington 1 , Michael Kemp 2 , Lauren McGarry 1 , Yu Chen 2 , M Elizabeth Stroupe 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-13 , DOI: 10.1038/s41467-020-14722-1 Joseph M Pennington 1 , Michael Kemp 2 , Lauren McGarry 1 , Yu Chen 2 , M Elizabeth Stroupe 1
Affiliation
|
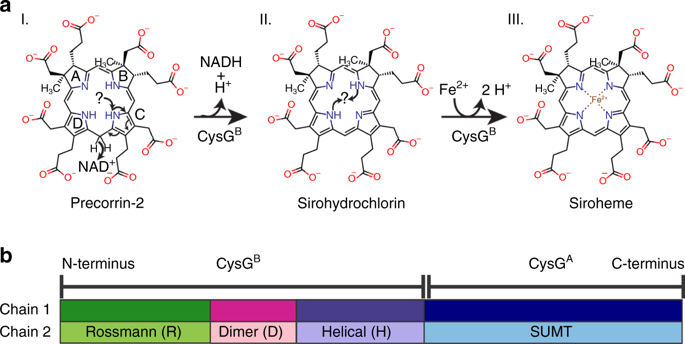
Siroheme is the central cofactor in a conserved class of sulfite and nitrite reductases that catalyze the six-electron reduction of sulfite to sulfide and nitrite to ammonia. In Salmonella enterica serovar Typhimurium, siroheme is produced by a trifunctional enzyme, siroheme synthase (CysG). A bifunctional active site that is distinct from its methyltransferase activity catalyzes the final two steps, NAD+-dependent dehydrogenation and iron chelation. How this active site performs such different chemistries is unknown. Here, we report the structures of CysG bound to precorrin-2, the initial substrate; sirohydrochlorin, the dehydrogenation product/chelation substrate; and a cobalt-sirohydrochlorin product. We identified binding poses for all three tetrapyrroles and tested the roles of specific amino acids in both activities to give insights into how a bifunctional active site catalyzes two different chemistries and acts as an iron-specific chelatase in the final step of siroheme synthesis.
中文翻译:

Siroheme合酶使底物在共同的活性位点中具有脱氢酶和螯合酶活性。
西罗血红素是一类保守的亚硫酸盐和亚硝酸盐还原酶中的主要辅助因子,亚硫酸盐和亚硝酸盐还原酶可催化将亚硫酸盐六价电子还原为硫化物,将亚硝酸盐还原为氨。在肠炎沙门氏菌血清型鼠伤寒沙门氏菌中,西罗血红素是由三功能酶-西罗血红素合酶(CysG)产生的。不同于其甲基转移酶活性的双功能活性位点催化最后两个步骤,NAD +依赖性脱氢和铁螯合。尚不清楚该活性位点如何执行这种不同的化学反应。在这里,我们报告绑定到precorrin-2,初始底物的CysG的结构;西罗霉素,脱氢产物/螯合底物;以及钴-西盐酸钴产品。
更新日期:2020-02-13
中文翻译:

Siroheme合酶使底物在共同的活性位点中具有脱氢酶和螯合酶活性。
西罗血红素是一类保守的亚硫酸盐和亚硝酸盐还原酶中的主要辅助因子,亚硫酸盐和亚硝酸盐还原酶可催化将亚硫酸盐六价电子还原为硫化物,将亚硝酸盐还原为氨。在肠炎沙门氏菌血清型鼠伤寒沙门氏菌中,西罗血红素是由三功能酶-西罗血红素合酶(CysG)产生的。不同于其甲基转移酶活性的双功能活性位点催化最后两个步骤,NAD +依赖性脱氢和铁螯合。尚不清楚该活性位点如何执行这种不同的化学反应。在这里,我们报告绑定到precorrin-2,初始底物的CysG的结构;西罗霉素,脱氢产物/螯合底物;以及钴-西盐酸钴产品。






























 京公网安备 11010802027423号
京公网安备 11010802027423号