当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Squaramide-Catalyzed Asymmetric Mannich Reactions of Azlactones with Isatin-Derived Ketimines: Access to α,β-Diamino Acid Derivatives Bearing Vicinal Quaternary Stereocenters.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-02-12 , DOI: 10.1021/acs.joc.9b03000 Zhanhuan Li 1 , Jingyi Peng 1 , Chonglong He 1 , Jianfeng Xu 1 , Hongjun Ren 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2020-02-12 , DOI: 10.1021/acs.joc.9b03000 Zhanhuan Li 1 , Jingyi Peng 1 , Chonglong He 1 , Jianfeng Xu 1 , Hongjun Ren 2
Affiliation
|
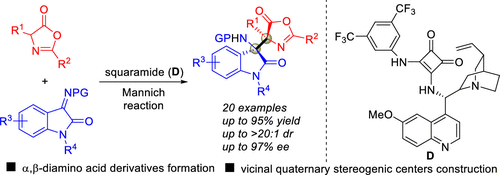
A highly stereoselective Mannich reaction of α-amino acid derived azlactones with isatin-derived ketimines enabled by a chiral bifunctional squaramide organocatalyst is reported, affording α,β-diamino acid derivatives bearing vicinal quaternary stereocenters in moderate to good yields (40-95%), moderate to excellent diastereoselectivities (3:1 → 20:1), and good to excellent enantioselectivities (66-97%). This reaction can be readily performed on gram scale, and the Mannich adduct could be easily converted to the corresponding α,β-diamino ester via simple operations.
中文翻译:

方苯二甲酰胺催化的丁二酮与Isatin衍生的Ketimines的不对称曼尼希反应:获得带有邻位四元立体中心的α,β-二氨基酸衍生物。
据报道,α-氨基酸衍生的内酯与手性双官能方酰胺有机催化剂可实现由靛红衍生的酮亚胺的高度立体选择性曼尼希反应,从而以中等至良好的收率(40-95%)提供具有邻位四级立体中心的α,β-二氨基酸衍生物。 ,中等至极好的非对映选择性(3:1→20:1)和良好至极好的对映选择性(66-97%)。该反应可以以克为单位容易地进行,并且曼尼希加合物可以通过简单的操作容易地转化为相应的α,β-二氨基酯。
更新日期:2020-02-19
中文翻译:

方苯二甲酰胺催化的丁二酮与Isatin衍生的Ketimines的不对称曼尼希反应:获得带有邻位四元立体中心的α,β-二氨基酸衍生物。
据报道,α-氨基酸衍生的内酯与手性双官能方酰胺有机催化剂可实现由靛红衍生的酮亚胺的高度立体选择性曼尼希反应,从而以中等至良好的收率(40-95%)提供具有邻位四级立体中心的α,β-二氨基酸衍生物。 ,中等至极好的非对映选择性(3:1→20:1)和良好至极好的对映选择性(66-97%)。该反应可以以克为单位容易地进行,并且曼尼希加合物可以通过简单的操作容易地转化为相应的α,β-二氨基酯。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号