当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluidic Multivalent Membrane Nanointerface Enables Synergetic Enrichment of Circulating Tumor Cells with High Efficiency and Viability
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-12 , DOI: 10.1021/jacs.9b13782
Lingling Wu 1 , Hongming Ding 2 , Xin Qu 3 , Xianai Shi 3 , Jianmin Yang 3 , Mengjiao Huang 4 , Jialu Zhang 1 , Huimin Zhang 1 , Jia Song 1 , Lin Zhu 4 , Yanling Song 1, 4 , Yuqiang Ma 5 , Chaoyong Yang 1, 4
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-02-12 , DOI: 10.1021/jacs.9b13782
Lingling Wu 1 , Hongming Ding 2 , Xin Qu 3 , Xianai Shi 3 , Jianmin Yang 3 , Mengjiao Huang 4 , Jialu Zhang 1 , Huimin Zhang 1 , Jia Song 1 , Lin Zhu 4 , Yanling Song 1, 4 , Yuqiang Ma 5 , Chaoyong Yang 1, 4
Affiliation
|
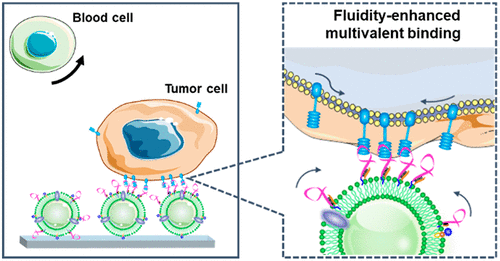
The ubiquitous biomembrane interface, with its dynamic lateral fluidity, allows membrane-bound components to rearrange and localize for high-affinity multivalent ligand-receptor interactions in diverse life activities. Inspired by this, we herein engineered a fluidic multivalent nanointerface by decorating a microfluidic chip with aptamer-functionalized leukocyte membrane nanovesicles for high-performance isolation of circulating tumor cells (CTCs). This fluidic biomimetic nanointerface with active recruitment-binding affords significant affinity enhancement by four orders of magnitude, exhibiting 7-fold higher capture efficiency compared to a monovalent aptamer functionalized-chip in blood. Meanwhile, this soft nanointerface inherits the biological benefits of a natural biomembrane, min-imizing background blood cell adsorption and maintaining excellent CTC viability (97.6%). Using the chip, CTCs were successfully detected in all cancer patient samples tested (17/17), suggesting the high potential of this fluidity-enhanced multivalent strategy in clinical applications. We expect this bioengineered interface strategy will lead to the design of innovative biomimetic platforms in the biomedical field by leveraging natural cell-cell interaction with a natural biomaterial.
中文翻译:

流体多价膜纳米界面能够高效、高效地协同富集循环肿瘤细胞
无处不在的生物膜界面具有动态横向流动性,允许膜结合成分重新排列和定位,以在不同的生命活动中实现高亲和力的多价配体 - 受体相互作用。受此启发,我们在此通过用适体功能化的白细胞膜纳米囊泡装饰微流控芯片设计了一种流体多价纳米界面,用于循环肿瘤细胞 (CTC) 的高性能分离。这种具有主动募集结合的流体仿生纳米界面提供了四个数量级的显着亲和力增强,与血液中的单价适体功能化芯片相比,其捕获效率提高了 7 倍。同时,这种柔软的纳米界面继承了天然生物膜的生物学益处,最大限度地减少背景血细胞吸附并保持出色的 CTC 活力 (97.6%)。使用该芯片,在所有测试的癌症患者样本中成功检测到 CTC (17/17),表明这种流动性增强的多价策略在临床应用中具有巨大潜力。我们预计这种生物工程接口策略将通过利用天然细胞与天然生物材料的天然细胞相互作用,导致生物医学领域创新仿生平台的设计。
更新日期:2020-02-12
中文翻译:

流体多价膜纳米界面能够高效、高效地协同富集循环肿瘤细胞
无处不在的生物膜界面具有动态横向流动性,允许膜结合成分重新排列和定位,以在不同的生命活动中实现高亲和力的多价配体 - 受体相互作用。受此启发,我们在此通过用适体功能化的白细胞膜纳米囊泡装饰微流控芯片设计了一种流体多价纳米界面,用于循环肿瘤细胞 (CTC) 的高性能分离。这种具有主动募集结合的流体仿生纳米界面提供了四个数量级的显着亲和力增强,与血液中的单价适体功能化芯片相比,其捕获效率提高了 7 倍。同时,这种柔软的纳米界面继承了天然生物膜的生物学益处,最大限度地减少背景血细胞吸附并保持出色的 CTC 活力 (97.6%)。使用该芯片,在所有测试的癌症患者样本中成功检测到 CTC (17/17),表明这种流动性增强的多价策略在临床应用中具有巨大潜力。我们预计这种生物工程接口策略将通过利用天然细胞与天然生物材料的天然细胞相互作用,导致生物医学领域创新仿生平台的设计。































 京公网安备 11010802027423号
京公网安备 11010802027423号