Nature ( IF 50.5 ) Pub Date : 2020-02-12 , DOI: 10.1038/s41586-020-2000-y
Kheewoong Baek 1 , David T Krist 1, 2 , J Rajan Prabu 1 , Spencer Hill 3 , Maren Klügel 1 , Lisa-Marie Neumaier 1 , Susanne von Gronau 1 , Gary Kleiger 3 , Brenda A Schulman 1
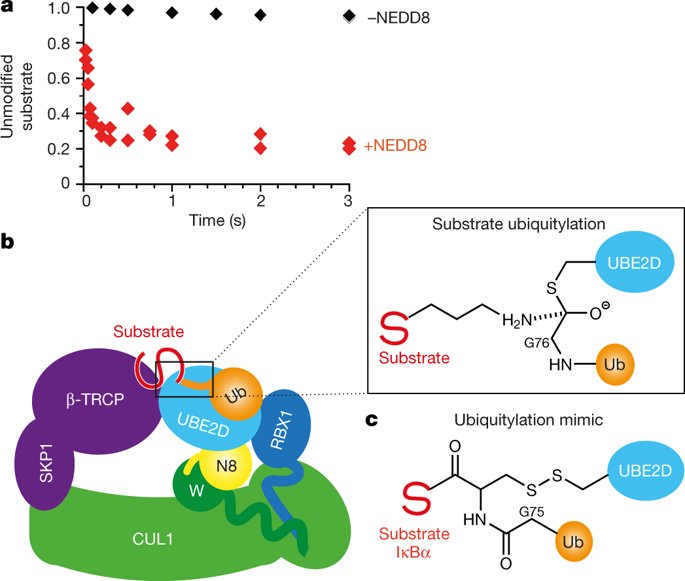
|
Eukaryotic cell biology depends on cullin–RING E3 ligase (CRL)-catalysed protein ubiquitylation1, which is tightly controlled by the modification of cullin with the ubiquitin-like protein NEDD82,3,4,5,6. However, how CRLs catalyse ubiquitylation, and the basis of NEDD8 activation, remain unknown. Here we report the cryo-electron microscopy structure of a chemically trapped complex that represents the ubiquitylation intermediate, in which the neddylated CRL1β-TRCP promotes the transfer of ubiquitin from the E2 ubiquitin-conjugating enzyme UBE2D to its recruited substrate, phosphorylated IκBα. NEDD8 acts as a nexus that binds disparate cullin elements and the RING-activated ubiquitin-linked UBE2D. Local structural remodelling of NEDD8 and large-scale movements of CRL domains converge to juxtapose the substrate and the ubiquitylation active site. These findings explain how a distinctive ubiquitin-like protein alters the functions of its targets, and show how numerous NEDD8-dependent interprotein interactions and conformational changes synergistically configure a catalytic CRL architecture that is both robust, to enable rapid ubiquitylation of the substrate, and fragile, to enable the subsequent functions of cullin–RING proteins.
中文翻译:

NEDD8 使多价 cullin-RING-UBE2D 泛素连接组装体成核
真核细胞生物学依赖于 cullin-RING E3 连接酶 (CRL) 催化的蛋白质泛素化1,其受 cullin 与泛素样蛋白 NEDD82,3,4,5,6 的修饰的严格控制。然而,CRL 如何催化泛素化以及 NEDD8 激活的基础仍然未知。在这里,我们报道了代表泛素化中间体的化学捕获复合物的冷冻电子显微镜结构,其中 neddylated CRL1β-TRCP 促进泛素从 E2 泛素结合酶 UBE2D 转移到其募集的底物磷酸化 IκBα。NEDD8 充当结合不同 cullin 元件和 RING 激活的泛素连接 UBE2D 的纽带。NEDD8 的局部结构重塑和 CRL 结构域的大规模运动汇聚在一起,将底物和泛素化活性位点并列。这些发现解释了独特的泛素样蛋白如何改变其靶标的功能,并展示了众多依赖性 NEDD8 的蛋白间相互作用和构象变化如何协同构建催化 CRL 结构,该结构既稳健,使底物快速泛素化,又脆弱,以实现 cullin-RING 蛋白的后续功能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号