当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Core structure dependence of cycloreversion dynamics in diarylethene analogs.
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9cp05797h Chana R Honick 1 , Garvin M Peters 1 , Jamie D Young 1 , John D Tovar 1 , Arthur E Bragg 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-01-23 , DOI: 10.1039/c9cp05797h Chana R Honick 1 , Garvin M Peters 1 , Jamie D Young 1 , John D Tovar 1 , Arthur E Bragg 1
Affiliation
|
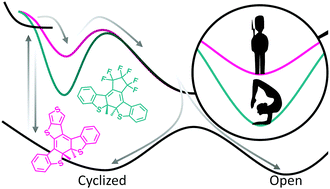
Diarylperfluorocyclopentenes are a well-characterized class of molecular photoswitches that undergo reversible photocyclization. The efficiency of cycloreversion (<∼30%), in particular, is known to be limited by a competition with excited-state deactivation by internal conversion that is strongly impacted by the electron-withdrawing/donating character of pendant aryl groups. Here we present a first study to determine how varied structural motifs for the core bridge group impact excited-state dynamics that control cycloreversion quantum yields. Specifically, we compare photophysical behaviors of 3,3'-(perfluorocyclopent-1-ene-1,2-diyl)bis(2-methylbenzo[b]thiophene) with diarylethene derivatives possessing the same benzo[b]thiophene pendant group but with a rigid 1-methyl-1H-pyrrole-2,5-dione and a rigid/aromatic thieno[3,4-b]thiophene bridge (TT) core bridge group. We find that the flexible perfluorocyclopentene core undergoes cycloreversion 3-4× slower than the rigid core photoswitches (9 vs. 2-3 ps in acetonitrile, 25 vs. 5-6 ps in cyclohexane) despite comparable cycloreversion quantum yields. To distinguish effects induced by bridge vs. pendant groups, we also studied a series of photoswitches with the same thieno[3,4-b]thiophene bridging group, but with varied pendant groups including 2,5-dimethylthiophene and 2-(3,5-bis(trifluoromethyl)phenyl)-5-methylthiophene. Analysis of temperature-dependent excited-state lifetimes and cycloreversion quantum yields reveals that both the rates of nonreactive internal conversion and reactive cycloreversion increase with greater structural rigidity of the core. This difference is attributed to smaller energy barriers on the excited-state potential energy surface for both reactive and non-reactive deactivation from the 21A electronic state relative to the flexible perfluorocyclopentene switch, implying that a rigid core results in a net shallower excited-state potential energy surface.
中文翻译:

二芳基乙烯类似物的环还原动力学的核心结构依赖性。
二芳基全氟环戊烯是一类很好的分子光开关,其经历可逆的光环化。特别地,已知环还原的效率(<〜30%)受到内部转化与激发态失活的竞争的限制,该竞争受芳基侧基的吸电子/给电子特性强烈影响。在这里,我们目前进行的第一项研究是确定核心桥基团的不同结构基序如何影响控制环还原量子产率的激发态动力学。具体来说,我们比较了3,3'-(全氟环戊-1-烯-1,2-二基)双(2-甲基苯并[b]噻吩)与具有相同苯并[b]噻吩侧基的二芳基乙烯衍生物的光物理行为刚性的1-甲基-1H-吡咯-2,5-二酮和刚性/芳香族噻吩并[3,4-b]噻吩桥(TT)核心桥基。我们发现柔性全氟环戊烯核的环还原速度比刚性核光电开关慢3-4倍(乙腈为9 vs. 2-3 ps,环己烷为25 vs. 5-6 ps),尽管环还原的量子产率相当。为了区分桥基和侧基引起的效应,我们还研究了一系列具有相同噻吩并[3,4-b]噻吩桥基,但侧基包括2,5-二甲基噻吩和2-(3, 5-双(三氟甲基)苯基)-5-甲基噻吩。对温度相关的激发态寿命和环回复量子产率的分析表明,非反应性内部转化率和反应性环回复率均随铁心结构刚度的增加而增加。
更新日期:2020-02-13
中文翻译:

二芳基乙烯类似物的环还原动力学的核心结构依赖性。
二芳基全氟环戊烯是一类很好的分子光开关,其经历可逆的光环化。特别地,已知环还原的效率(<〜30%)受到内部转化与激发态失活的竞争的限制,该竞争受芳基侧基的吸电子/给电子特性强烈影响。在这里,我们目前进行的第一项研究是确定核心桥基团的不同结构基序如何影响控制环还原量子产率的激发态动力学。具体来说,我们比较了3,3'-(全氟环戊-1-烯-1,2-二基)双(2-甲基苯并[b]噻吩)与具有相同苯并[b]噻吩侧基的二芳基乙烯衍生物的光物理行为刚性的1-甲基-1H-吡咯-2,5-二酮和刚性/芳香族噻吩并[3,4-b]噻吩桥(TT)核心桥基。我们发现柔性全氟环戊烯核的环还原速度比刚性核光电开关慢3-4倍(乙腈为9 vs. 2-3 ps,环己烷为25 vs. 5-6 ps),尽管环还原的量子产率相当。为了区分桥基和侧基引起的效应,我们还研究了一系列具有相同噻吩并[3,4-b]噻吩桥基,但侧基包括2,5-二甲基噻吩和2-(3, 5-双(三氟甲基)苯基)-5-甲基噻吩。对温度相关的激发态寿命和环回复量子产率的分析表明,非反应性内部转化率和反应性环回复率均随铁心结构刚度的增加而增加。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号