当前位置:
X-MOL 学术
›
J. Electroanal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical synthesis of thiazoles from 2,5-dihydrothiazolines mediated by bromide/tetrachlorohydroquinone dual redox catalysts
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113941 Yong Li , Cao-Cao Sun , Cheng-Chu Zeng
Journal of Electroanalytical Chemistry ( IF 4.1 ) Pub Date : 2020-03-01 , DOI: 10.1016/j.jelechem.2020.113941 Yong Li , Cao-Cao Sun , Cheng-Chu Zeng
|
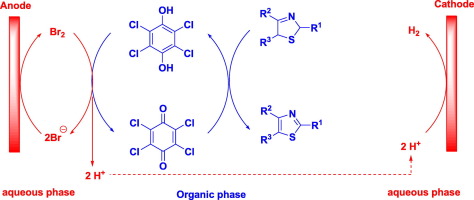
Abstract An efficient protocol for the electrochemical synthesis of thiazole derivatives has been developed. Initially, cyclic voltammetry (CV) measure and preparative electrolysis were performed to examine that halide ions and tetrachlorohydroquinone (TCHQ) are able to serve as redox catalysts for the transformation of TCHQ to tetrachloroquinone (TCQ) and 2,5-dihydrothiazoles to thiazoles, respectively. Next, a combination of bromide ion and TCHQ was employed successfully for the electrochemical synthesis of several representative thiazole derivatives in moderate to good yields. Finally, a possible reaction mechanism is proposed. The work may provide a practical protocol for the electrochemical synthesis of TCQ and thiazoles.
中文翻译:

溴化物/四氯氢醌双氧化还原催化剂介导的2,5-二氢噻唑啉电化学合成噻唑
摘要 开发了一种有效的噻唑衍生物电化学合成方案。最初,进行循环伏安法 (CV) 测量和制备电解以检查卤离子和四氯氢醌 (TCHQ) 能够作为氧化还原催化剂,分别将 TCHQ 转化为四氯醌 (TCQ) 和 2,5-二氢噻唑转化为噻唑. 接下来,溴离子和 TCHQ 的组合被成功用于电化学合成几种代表性的噻唑衍生物,产率中等至良好。最后,提出了可能的反应机理。该工作可能为 TCQ 和噻唑的电化学合成提供实用的方案。
更新日期:2020-03-01
中文翻译:

溴化物/四氯氢醌双氧化还原催化剂介导的2,5-二氢噻唑啉电化学合成噻唑
摘要 开发了一种有效的噻唑衍生物电化学合成方案。最初,进行循环伏安法 (CV) 测量和制备电解以检查卤离子和四氯氢醌 (TCHQ) 能够作为氧化还原催化剂,分别将 TCHQ 转化为四氯醌 (TCQ) 和 2,5-二氢噻唑转化为噻唑. 接下来,溴离子和 TCHQ 的组合被成功用于电化学合成几种代表性的噻唑衍生物,产率中等至良好。最后,提出了可能的反应机理。该工作可能为 TCQ 和噻唑的电化学合成提供实用的方案。






























 京公网安备 11010802027423号
京公网安备 11010802027423号