当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Spirocyclic Phosphoric Acid‐Catalyzed Synthesis of 4‐Alkyl‐3,4‐dihydropyrimidin‐2(1H)‐one Derivatives by Asymmetric Biginelli Reactions
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-20 , DOI: 10.1002/ajoc.201900718 Yongbiao Guo 1 , Zhenhua Gao 1 , Kang Wang 1 , Junchen Li 1 , Xiaojing Bi 1 , Lei Guo 1 , Haibo Liu 1 , Enxue Shi 1 , Junhua Xiao 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-02-20 , DOI: 10.1002/ajoc.201900718 Yongbiao Guo 1 , Zhenhua Gao 1 , Kang Wang 1 , Junchen Li 1 , Xiaojing Bi 1 , Lei Guo 1 , Haibo Liu 1 , Enxue Shi 1 , Junhua Xiao 1
Affiliation
|
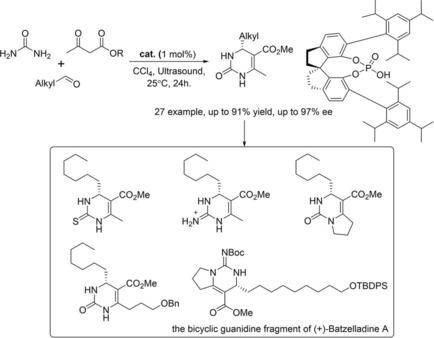
A chiral spirocyclic phosphoric acid‐catalyzed asymmetric Biginelli reaction based on aliphatic aldehydes has been realized for the synthesis of biologically important and synthetically useful 4‐alkyl‐3,4‐dihydropyrimidin‐2‐(1H)‐one (4‐alkyl−DHPM) skeletons in good yield and with high levels of enantioinduction. The utility of this transformation was further demonstrated by direct thionation and C−C/C−N bond construction to provide a series of 4‐alkyl−DHPMs derivatives without any loss of optical activity, providing streamlined access to the formal synthesis of Batzelladine A.
中文翻译:

手性螺环磷酸催化不对称Biginelli反应合成4-烷基-3,4-二氢嘧啶-2(1H)-one衍生物
已经实现了基于脂族醛的手性螺环磷酸催化的不对称Biginelli反应,用于合成生物学上重要且可合成的4-烷基-3,4-二氢嘧啶-2-(1H)-一(4-烷基-DHPM)骨架,产量高,对映体诱导水平高。通过直接硫磺化和C-C / C-N键结构进一步证明了这种转化的效用,从而提供了一系列4-烷基-DHPMs衍生物,而没有任何光学活性的损失,为简化的Batzelladine A的正式合成提供了途径。
更新日期:2020-04-21
中文翻译:

手性螺环磷酸催化不对称Biginelli反应合成4-烷基-3,4-二氢嘧啶-2(1H)-one衍生物
已经实现了基于脂族醛的手性螺环磷酸催化的不对称Biginelli反应,用于合成生物学上重要且可合成的4-烷基-3,4-二氢嘧啶-2-(1H)-一(4-烷基-DHPM)骨架,产量高,对映体诱导水平高。通过直接硫磺化和C-C / C-N键结构进一步证明了这种转化的效用,从而提供了一系列4-烷基-DHPMs衍生物,而没有任何光学活性的损失,为简化的Batzelladine A的正式合成提供了途径。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号