Applied Catalysis B: Environment and Energy ( IF 20.2 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.apcatb.2020.118747 Yifan Li , Chang Chen , Rui Cao , Ziwei Pan , Hua He , Kebin Zhou
|
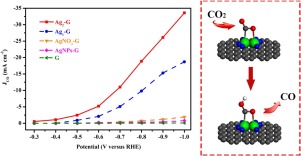
Electrochemical reduction of CO2 into value-added carbon compounds offers a promising strategy to mitigate global warming, but present challenges for chemistry due to the poor selectivity and stability of electrocatalysts. In this work, we report a dual-atom Ag2/graphene catalyst featuring well-defined AgN3-AgN3 active site for CO2 electrochemical reduction. This dual-atom catalyst can drive CO2 reduction reaction at a potential as high as -0.25 V, and exhibit excellent CO Faradic efficiency up to 93.4% with a current density of 11.87 mA cm-2 at -0.7 V and long-term stability, far surpassing the single-atom Ag1/graphene and the traditional silver nanoparticle catalysts. DFT calculations reveal that the dual-atom Ag site lowers the barrier for the formation of *COOH by stabilizing the *CO2 through the concomitant interactions with the C and an O atom of CO2, resulting in excellent catalytic performance.
中文翻译:

双原子Ag 2 /石墨烯催化剂,可将CO 2有效电还原为CO
将CO 2电化学还原成增值碳化合物为缓解全球变暖提供了一种有前途的策略,但由于电催化剂的选择性和稳定性差,因此在化学方面面临挑战。在这项工作中,我们报告了一种双原子Ag 2 /石墨烯催化剂,其特征在于用于CO 2电化学还原的明确定义的AgN 3 -AgN 3活性位。该双原子催化剂可在-0.25 V的高电位下驱动CO 2还原反应,并在-0.7 V时具有11.87 mA cm -2的电流密度,并具有高达93.4%的出色CO Faradic效率和长期稳定性。 ,远远超过单原子Ag 1/石墨烯和传统的银纳米颗粒催化剂。DFT计算表明,双原子的Ag现场通过稳定* CO降低了* COOH形成的屏障2通过与C和CO的O原子的伴随的交互2,从而导致优异的催化性能。

































 京公网安备 11010802027423号
京公网安备 11010802027423号