当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Biotransformation Changes Bioaccumulation and Toxicity of Diclofenac in Aquatic Organisms.
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.est.9b07127 Qiuguo Fu 1 , Davide Fedrizzi 1 , Verena Kosfeld 2, 3 , Christian Schlechtriem 2, 3 , Vera Ganz 1, 4 , Samuel Derrer 1 , Daniel Rentsch 5 , Juliane Hollender 1, 4
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2020-02-10 , DOI: 10.1021/acs.est.9b07127 Qiuguo Fu 1 , Davide Fedrizzi 1 , Verena Kosfeld 2, 3 , Christian Schlechtriem 2, 3 , Vera Ganz 1, 4 , Samuel Derrer 1 , Daniel Rentsch 5 , Juliane Hollender 1, 4
Affiliation
|
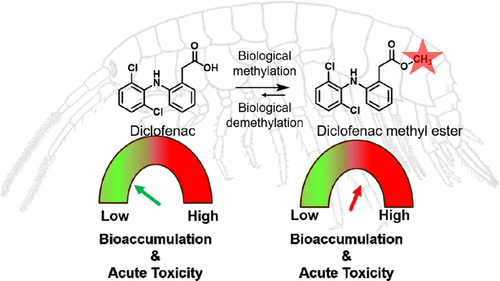
Biotransformation plays a crucial role in regulating the bioaccumulation potential and toxicity of organic compounds in organisms but is, in general, poorly understood for emerging contaminants. Here we have used diclofenac as a model compound to study the impact of biotransformation on the bioaccumulation potential and toxicity in two keystone aquatic invertebrates: Gammarus pulex and Hyalella azteca. In both species, diclofenac was transformed into several oxidation products and conjugates, including two novel products, i.e. diclofenac taurine conjugate (DCF-M403) and unexpected diclofenac methyl ester (DCF-M310.03). The ratios of biotransformation products to parent compound were 12-17 for DCF-M403 and 0.01-0.7 for DCF-M310.03 after 24 h exposure. Bioconcentration factors (BCFs) of diclofenac were 0.5 and 3.2 L kg ww-1 in H. azteca and G. pulex, respectively, whereas BCFs of DCF-M310.03 was 164.5 L kg ww-1 and 104.7 L kg ww-1, respectively, representing a 25 to 110-fold increase. Acute toxicity of DCF-M310.03 was also higher than the parent compound in both species, which correlated well with the increased bioconcentration potential. The LC50 of diclofenac in H. azteca was 216 mg L-1, while that of metabolite DCF-M310.03 was reduced to only 0.53 mg L-1, representing a 430-fold increase in acute toxicity compared to diclofenac. DCF-M403 is less toxic than its parent compound towards H. azteca, which may be linked to its slightly lower hydrophobicity. Furthermore, the transformation of diclofenac to its methyl ester derivative was explored in crude invertebrate extracts spiked with an S-adenosylmethionine cofactor, revealing possible catalysis by an S-adenosylmethionine-dependent -carboxylic acid methyltransferase. Methylation of diclofenac was further detected in fish hepatocytes and human urine, indicating a broader relevance. Therefore, potentially methylated metabolites of polar contaminants should be considered for a comprehensive risk assessment in the future.
中文翻译:

生物转化改变了双氯芬酸在水生生物中的生物蓄积性和毒性。
生物转化在调节有机物中有机化合物的生物积累潜力和毒性方面起着至关重要的作用,但是,对于新出现的污染物,总体上了解甚少。在这里,我们已经使用双氯芬酸作为模型化合物来研究生物转化对两种主要水生无脊椎动物Gammarus pulex和Hyalella azteca的生物积累潜力和毒性的影响。在这两个物种中,双氯芬酸被转化为几种氧化产物和结合物,包括两种新产物,即双氯芬酸牛磺酸结合物(DCF-M403)和意外的双氯芬酸甲酯(DCF-M310.03)。暴露24小时后,DCF-M403的生物转化产物与母体化合物的比率为12-17,DCF-M310.03的生物转化产物与母体化合物的比率为0.01-0.7。双氯芬酸在阿兹台克人和普勒克氏菌中的生物浓缩因子(BCF)为0.5和3.2 L kg ww-1,DCF-M310.03的BCF分别为164.5 L kg ww-1和104.7 L kg ww-1,代表增加了25到110倍。在两个物种中,DCF-M310.03的急性毒性也都高于母体化合物,这与增加的生物富集潜力密切相关。双氯芬酸在双歧杆菌中的LC50为216 mg L-1,而代谢产物DCF-M310.03的LC50降低至仅0.53 mg L-1,与双氯芬酸相比,急性毒性增加了430倍。DCF-M403比其母体化合物对阿兹台克人的毒性更小,这可能与其疏水性稍低有关。此外,在掺有S-腺苷甲硫氨酸辅因子的无脊椎动物粗提物中探索了双氯芬酸向其甲酯衍生物的转化,揭示了S-腺苷甲硫氨酸依赖性-羧酸甲基转移酶可能的催化作用。在鱼肝细胞和人尿中进一步检测到了双氯芬酸的甲基化,表明其相关性更广。因此,将来应考虑对极性污染物的潜在甲基化代谢产物进行全面的风险评估。
更新日期:2020-03-12
中文翻译:

生物转化改变了双氯芬酸在水生生物中的生物蓄积性和毒性。
生物转化在调节有机物中有机化合物的生物积累潜力和毒性方面起着至关重要的作用,但是,对于新出现的污染物,总体上了解甚少。在这里,我们已经使用双氯芬酸作为模型化合物来研究生物转化对两种主要水生无脊椎动物Gammarus pulex和Hyalella azteca的生物积累潜力和毒性的影响。在这两个物种中,双氯芬酸被转化为几种氧化产物和结合物,包括两种新产物,即双氯芬酸牛磺酸结合物(DCF-M403)和意外的双氯芬酸甲酯(DCF-M310.03)。暴露24小时后,DCF-M403的生物转化产物与母体化合物的比率为12-17,DCF-M310.03的生物转化产物与母体化合物的比率为0.01-0.7。双氯芬酸在阿兹台克人和普勒克氏菌中的生物浓缩因子(BCF)为0.5和3.2 L kg ww-1,DCF-M310.03的BCF分别为164.5 L kg ww-1和104.7 L kg ww-1,代表增加了25到110倍。在两个物种中,DCF-M310.03的急性毒性也都高于母体化合物,这与增加的生物富集潜力密切相关。双氯芬酸在双歧杆菌中的LC50为216 mg L-1,而代谢产物DCF-M310.03的LC50降低至仅0.53 mg L-1,与双氯芬酸相比,急性毒性增加了430倍。DCF-M403比其母体化合物对阿兹台克人的毒性更小,这可能与其疏水性稍低有关。此外,在掺有S-腺苷甲硫氨酸辅因子的无脊椎动物粗提物中探索了双氯芬酸向其甲酯衍生物的转化,揭示了S-腺苷甲硫氨酸依赖性-羧酸甲基转移酶可能的催化作用。在鱼肝细胞和人尿中进一步检测到了双氯芬酸的甲基化,表明其相关性更广。因此,将来应考虑对极性污染物的潜在甲基化代谢产物进行全面的风险评估。






























 京公网安备 11010802027423号
京公网安备 11010802027423号