当前位置:
X-MOL 学术
›
Int. J. Biol. Macromol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The binding mechanism of nitroreductase fluorescent probe: Active pocket deformation and intramolecular hydrogen bonds.
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ijbiomac.2020.02.084 Min Zhu 1 , Rui Rui Liu 1 , Hong Lin Zhai 1 , Ya Jie Meng 1 , Lu Han 1 , Cui Ling Ren 1
International Journal of Biological Macromolecules ( IF 7.7 ) Pub Date : 2020-02-10 , DOI: 10.1016/j.ijbiomac.2020.02.084 Min Zhu 1 , Rui Rui Liu 1 , Hong Lin Zhai 1 , Ya Jie Meng 1 , Lu Han 1 , Cui Ling Ren 1
Affiliation
|
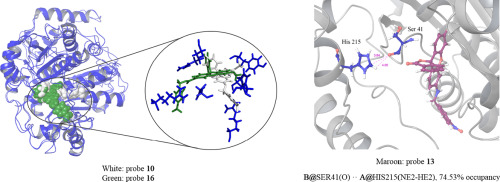
Nitroreductase (NTR), a member of the flavoenzyme family, could react with nicotinamide adenine dinucleotide by reducing nitro to amino at hypoxic tumor, which can be monitored by some fluorescent probes in vivo. Here, molecular docking and molecular dynamics simulation techniques were used to explore the molecular mechanisms between NTR and probes. The results showed that formation of hydrogen bond in 1F5V-13 between A@His215 and B@Ser41 with 74.53% occupancy might be the main reason for the decrease of probe fluorescence emission in experiment. Moreover, Probe 16 was rotated by nearly 60 degrees with respect to the position of other probes in protein binding pocket, deforming the protein active pocket, changing the hydrogen bond formation, which leads to the fluorescence performance of 16 with electron donor and electron acceptor groups was superior to other probes in experiment. The deformation of protein active pocket and the formation of intramolecular hydrogen bonds revealed the difference in performance of NTR fluorescent probe at molecular level, which provide theoretical guidance for latter design of fluorescent probes with better performance.
中文翻译:

硝基还原酶荧光探针的结合机理:活性口袋变形和分子内氢键。
flavoenzyme家族的一员,硝基还原酶(NTR)可以通过将缺氧肿瘤上的硝基还原为氨基来与烟酰胺腺嘌呤二核苷酸反应,这可以通过体内的某些荧光探针进行监测。在这里,分子对接和分子动力学模拟技术被用来探索NTR和探针之间的分子机制。结果表明,A @ His215和B @ Ser41之间1F5V-13中氢键的形成占74.53%,这可能是实验中探针荧光发射降低的主要原因。此外,探针16相对于蛋白质结合袋中其他探针的位置旋转了近60度,使蛋白质活性袋变形,改变了氢键的形成,这使得具有电子给体和电子受体基团的16的荧光性能在实验中优于其他探针。蛋白质活性口袋的变形和分子内氢键的形成揭示了NTR荧光探针在分子水平上的性能差异,这为以后设计性能更好的荧光探针提供了理论指导。
更新日期:2020-02-10
中文翻译:

硝基还原酶荧光探针的结合机理:活性口袋变形和分子内氢键。
flavoenzyme家族的一员,硝基还原酶(NTR)可以通过将缺氧肿瘤上的硝基还原为氨基来与烟酰胺腺嘌呤二核苷酸反应,这可以通过体内的某些荧光探针进行监测。在这里,分子对接和分子动力学模拟技术被用来探索NTR和探针之间的分子机制。结果表明,A @ His215和B @ Ser41之间1F5V-13中氢键的形成占74.53%,这可能是实验中探针荧光发射降低的主要原因。此外,探针16相对于蛋白质结合袋中其他探针的位置旋转了近60度,使蛋白质活性袋变形,改变了氢键的形成,这使得具有电子给体和电子受体基团的16的荧光性能在实验中优于其他探针。蛋白质活性口袋的变形和分子内氢键的形成揭示了NTR荧光探针在分子水平上的性能差异,这为以后设计性能更好的荧光探针提供了理论指导。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号