当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with ferric chloride and dipivaloylmethane as the catalytic system
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/24 , DOI: 10.1039/c9gc04126e Jian Lv 1, 2, 3, 4, 5 , Yu Liu 1, 2, 3, 4, 5 , Jia-Jia Zhu 1, 2, 3, 4, 5 , Dapeng Zou 6, 7, 8, 9 , Hai Dong 1, 2, 3, 4, 5
Green Chemistry ( IF 9.3 ) Pub Date : 2020/01/24 , DOI: 10.1039/c9gc04126e Jian Lv 1, 2, 3, 4, 5 , Yu Liu 1, 2, 3, 4, 5 , Jia-Jia Zhu 1, 2, 3, 4, 5 , Dapeng Zou 6, 7, 8, 9 , Hai Dong 1, 2, 3, 4, 5
Affiliation
|
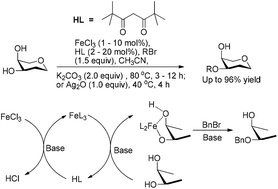
In this study, we reported the regio/site-selective alkylation of substrates containing a cis-, 1,2- or 1,3-diol with FeCl3 as a key catalyst. A catalytic system consisting of FeCl3 (0.01–0.1 equiv.) and dipivaloylmethane (FeCl3/dipivaloylmethane = 1/2) was used to catalyze the alkylation in the presence of a base. The produced selectivities and isolated yields were similar to those obtained by methods using the same amount of FeL3 (L = acylacetone ligand) as the catalyst in most cases. The previously reported FeL3 catalysts for alkylation are not commercially available and have to be synthesized prior to use. In contrast, FeCl3 and dipivaloylmethane (Hdipm) are very common and inexpensive nontoxic reagents in the lab, thereby making the method much greener and easier to handle. Mechanism studies confirmed for the first time that FeCl3 initially reacts with two equivalents of Hdipm to form [Fe(dipm)3] in the presence of a base in acetonitrile, followed by the formation of a five or six-membered ring intermediate between [Fe(dipm)3] and two hydroxyl groups of the substrate. A subsequent reaction between the cyclic intermediate and the alkylating agent results in selective alkylation of the substrate.
中文翻译:

含有顺式,1,2-或1,3-二醇的底物的区域/部位选择性烷基化,以氯化铁和二戊酰甲烷作为催化体系
在这项研究中,我们报道了含有顺式-,1,2-或1,3-二醇的底物的区域/位置选择性烷基化,其中FeCl 3为关键催化剂。由FeCl 3(0.01-0.1当量)和二戊酰基甲烷(FeCl 3 / dipivaloylmethane = 1/2)组成的催化体系用于在存在碱的情况下催化烷基化。在大多数情况下,所产生的选择性和分离的产率与使用相同量的FeL 3(L =酰基丙酮配体)作为催化剂的方法所获得的相似。先前报道的用于烷基化的FeL 3催化剂不可商购,并且必须在使用前合成。相反,FeCl 3苯二甲酰甲烷(Hdipm)和二甲戊酰甲烷(Hdipm)是实验室中非常普遍且廉价的无毒试剂,因此使该方法更加绿色且易于操作。机理研究首次证实,FeCl 3最初在乙腈中存在碱的情况下与两当量的Hdipm反应形成[Fe(dipm)3 ],然后在[[]之间形成五或六元环中间体。 Fe(dipm)3 ]和底物的两个羟基。环状中间体和烷基化剂之间的后续反应导致底物的选择性烷基化。
更新日期:2020-02-24
中文翻译:

含有顺式,1,2-或1,3-二醇的底物的区域/部位选择性烷基化,以氯化铁和二戊酰甲烷作为催化体系
在这项研究中,我们报道了含有顺式-,1,2-或1,3-二醇的底物的区域/位置选择性烷基化,其中FeCl 3为关键催化剂。由FeCl 3(0.01-0.1当量)和二戊酰基甲烷(FeCl 3 / dipivaloylmethane = 1/2)组成的催化体系用于在存在碱的情况下催化烷基化。在大多数情况下,所产生的选择性和分离的产率与使用相同量的FeL 3(L =酰基丙酮配体)作为催化剂的方法所获得的相似。先前报道的用于烷基化的FeL 3催化剂不可商购,并且必须在使用前合成。相反,FeCl 3苯二甲酰甲烷(Hdipm)和二甲戊酰甲烷(Hdipm)是实验室中非常普遍且廉价的无毒试剂,因此使该方法更加绿色且易于操作。机理研究首次证实,FeCl 3最初在乙腈中存在碱的情况下与两当量的Hdipm反应形成[Fe(dipm)3 ],然后在[[]之间形成五或六元环中间体。 Fe(dipm)3 ]和底物的两个羟基。环状中间体和烷基化剂之间的后续反应导致底物的选择性烷基化。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号