当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unravelling the mechanism of the organocatalyzed aminolytic kinetic resolution of α-nitroepoxides: a theoretical study
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/16 , DOI: 10.1039/c9cy01800j Amedeo Capobianco 1, 2, 3, 4 , Sara Meninno 1, 2, 3, 4 , Alessandra Lattanzi 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020/01/16 , DOI: 10.1039/c9cy01800j Amedeo Capobianco 1, 2, 3, 4 , Sara Meninno 1, 2, 3, 4 , Alessandra Lattanzi 1, 2, 3, 4
Affiliation
|
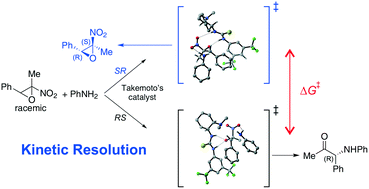
The aminolytic kinetic resolution of 2-methyl-2-nitro-3-phenyl oxirane with aniline was investigated by quantum chemical computations. It has been assessed that the reaction proceeds via a concerted ring opening/elimination of nitrous acid mechanism. The results are in agreement with the experimental findings when using the Takemoto's catalyst, which is estimated to perform in a highly efficient manner in the kinetic resolution of this class of epoxides in the absence of any background reaction. H-Bonding, π–π and CH/π interactions appear to make a significant contribution to the formation of a ternary complex and a transition state, which preferentially facilitates the reaction of the (2R,3S)-nitroepoxide to the α-amino ketone, leaving (2S,3R)-nitroepoxide unreacted.
中文翻译:

阐明α-硝基环氧化物有机催化氨基分解动力学拆分的机理:理论研究
通过量子化学计算研究了2-甲基-2-硝基-3-苯基环氧乙烷与苯胺的氨基分解动力学拆分。已经估计该反应通过协调的开环/亚硝酸消除机制进行。该结果与使用Takemoto催化剂时的实验结果相符,据估计,在没有任何背景反应的情况下,该催化剂可以高效地实现此类环氧化物的动力学拆分。H键,π–π和CH /π相互作用似乎对三元络合物和过渡态的形成做出了重要贡献,这优先促进了(2 R,3 S)-硝基环氧化物与α-氨基酮,残留(2 S,3 R)-硝基环氧化物未反应。
更新日期:2020-03-09
中文翻译:

阐明α-硝基环氧化物有机催化氨基分解动力学拆分的机理:理论研究
通过量子化学计算研究了2-甲基-2-硝基-3-苯基环氧乙烷与苯胺的氨基分解动力学拆分。已经估计该反应通过协调的开环/亚硝酸消除机制进行。该结果与使用Takemoto催化剂时的实验结果相符,据估计,在没有任何背景反应的情况下,该催化剂可以高效地实现此类环氧化物的动力学拆分。H键,π–π和CH /π相互作用似乎对三元络合物和过渡态的形成做出了重要贡献,这优先促进了(2 R,3 S)-硝基环氧化物与α-氨基酮,残留(2 S,3 R)-硝基环氧化物未反应。































 京公网安备 11010802027423号
京公网安备 11010802027423号