当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand-promoted cobalt-catalyzed radical hydroamination of alkenes.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-07 , DOI: 10.1038/s41467-020-14459-x
Xuzhong Shen 1 , Xu Chen 1 , Jieping Chen 1 , Yufeng Sun 1 , Zhaoyang Cheng 1 , Zhan Lu 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-07 , DOI: 10.1038/s41467-020-14459-x
Xuzhong Shen 1 , Xu Chen 1 , Jieping Chen 1 , Yufeng Sun 1 , Zhaoyang Cheng 1 , Zhan Lu 1
Affiliation
|
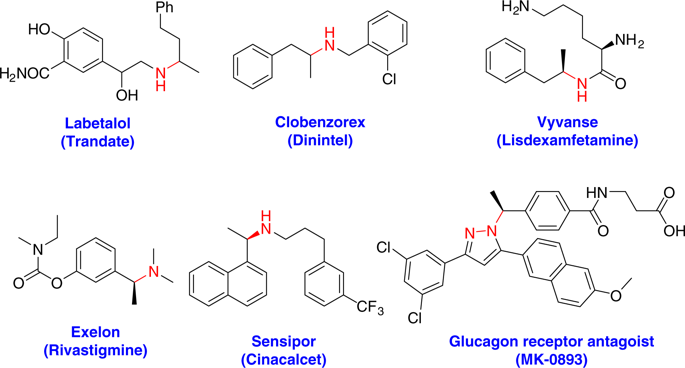
Highly regio- and enantioselective intermolecular hydroamination of alkenes is a challenging process potentially leading to valuable chiral amines. Hydroamination of alkenes via metal-catalyzed hydrogen atom transfer (HAT) with good regioselectivity and functional group tolerance has been reported, however, high enantioselectivity has not been achieved due to the lack of suitable ligands. Here we report a ligand-promoted cobalt-catalyzed Markovnikov-type selective radical hydroamination of alkenes with diazo compounds. This operationally simple protocol uses unsymmetric NNN-tridentate (UNT) ligand, readily available alkenes and hydrosilanes to construct hydrazones with good functional group tolerance. The hydrazones can undergo nitrogen-nitrogen bond cleavage smoothly to deliver valuable amine derivatives. Additionally, asymmetric intermolecular hydroamination of unactivated aliphatic terminal alkenes using chiral N-imidazolinylphenyl 8-aminoquinoline (IPAQ) ligands has also been achieved to afford chiral amine derivatives with good enantioselectivities.
中文翻译:

配体促进的钴催化的烯烃的自由基加氢胺化。
烯烃的高度区域和对映选择性分子间加氢胺化是一个具有挑战性的过程,有可能导致产生有价值的手性胺。已经报道了通过具有良好的区域选择性和官能团耐受性的金属催化的氢原子转移(HAT)对烯烃进行的加氢胺化,但是由于缺乏合适的配体,因此尚未实现高对映选择性。在这里我们报告了配体促进的钴与重氮化合物的烯烃的钴催化的马尔科夫尼科夫型选择性自由基加氢胺化反应。该操作简单的协议使用不对称的NNN三齿(UNT)配体,易于获得的烯烃和氢化硅烷来构建具有良好官能团耐受性的。可以顺利进行氮-氮键裂解,以提供有价值的胺衍生物。另外,
更新日期:2020-02-07
中文翻译:

配体促进的钴催化的烯烃的自由基加氢胺化。
烯烃的高度区域和对映选择性分子间加氢胺化是一个具有挑战性的过程,有可能导致产生有价值的手性胺。已经报道了通过具有良好的区域选择性和官能团耐受性的金属催化的氢原子转移(HAT)对烯烃进行的加氢胺化,但是由于缺乏合适的配体,因此尚未实现高对映选择性。在这里我们报告了配体促进的钴与重氮化合物的烯烃的钴催化的马尔科夫尼科夫型选择性自由基加氢胺化反应。该操作简单的协议使用不对称的NNN三齿(UNT)配体,易于获得的烯烃和氢化硅烷来构建具有良好官能团耐受性的。可以顺利进行氮-氮键裂解,以提供有价值的胺衍生物。另外,

































 京公网安备 11010802027423号
京公网安备 11010802027423号