当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TBC1D9 regulates TBK1 activation through Ca2+ signaling in selective autophagy.
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-07 , DOI: 10.1038/s41467-020-14533-4
Takashi Nozawa 1 , Shunsuke Sano 1 , Atsuko Minowa-Nozawa 1 , Hirotaka Toh 1 , Shintaro Nakajima 2, 3 , Kazunori Murase 1 , Chihiro Aikawa 1 , Ichiro Nakagawa 1
Nature Communications ( IF 14.7 ) Pub Date : 2020-02-07 , DOI: 10.1038/s41467-020-14533-4
Takashi Nozawa 1 , Shunsuke Sano 1 , Atsuko Minowa-Nozawa 1 , Hirotaka Toh 1 , Shintaro Nakajima 2, 3 , Kazunori Murase 1 , Chihiro Aikawa 1 , Ichiro Nakagawa 1
Affiliation
|
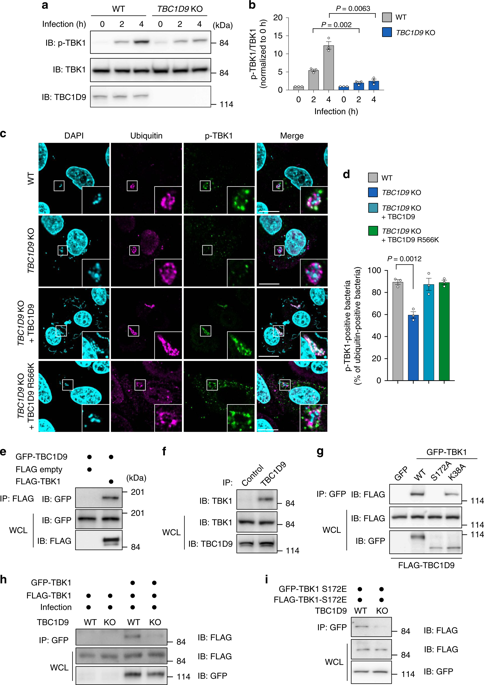
Invading microbial pathogens can be eliminated selectively by xenophagy. Ubiquitin-mediated autophagy receptors are phosphorylated by TANK-binding kinase 1 (TBK1) and recruited to ubiquitinated bacteria to facilitate autophagosome formation during xenophagy, but the molecular mechanism underlying TBK1 activation in response to microbial infection is not clear. Here, we show that bacterial infection increases Ca2+ levels to activate TBK1 for xenophagy via the Ca2+-binding protein TBC1 domain family member 9 (TBC1D9). Mechanistically, the ubiquitin-binding region (UBR) and Ca2+-binding motif of TBC1D9 mediate its binding with ubiquitin-positive bacteria, and TBC1D9 knockout suppresses TBK1 activation and subsequent recruitment of the ULK1 complex. Treatment with a Ca2+ chelator impairs TBC1D9-ubiquitin interactions and TBK1 activation during xenophagy. TBC1D9 is also recruited to damaged mitochondria through its UBR and Ca2+-binding motif, and is required for TBK1 activation during mitophagy. These results indicate that TBC1D9 controls TBK1 activation during xenophagy and mitophagy through Ca2+-dependent ubiquitin-recognition.
中文翻译:

TBC1D9通过选择性自噬中的Ca2 +信号传导调节TBK1激活。
可以通过异种吞噬选择性地消除入侵的微生物病原体。泛素介导的自噬受体被TANK结合激酶1(TBK1)磷酸化,并被募集到泛素化细菌以促进异种吞噬过程中自噬体的形成,但尚不清楚TBK1响应微生物感染而激活的分子机制。在这里,我们显示细菌感染通过Ca2 +结合蛋白TBC1域家族成员9(TBC1D9)增加了Ca2 +的水平以激活TBK1进行吞噬。从机械上讲,TBC1D9的泛素结合区(UBR)和Ca2 +结合基序介导其与泛素阳性细菌的结合,而TBC1D9敲除可抑制TBK1激活和随后的ULK1复合物募集。用Ca2 +螯合剂处理会损害异种吞噬过程中的TBC1D9-泛素相互作用和TBK1激活。TBC1D9还通过其UBR和Ca2 +结合基序募集到受损的线粒体,并且在线粒体吞噬过程中TBK1激活是必需的。这些结果表明,TBC1D9通过Ca2 +依赖性泛素识别控制异种和线粒体过程中的TBK1激活。
更新日期:2020-02-07
中文翻译:

TBC1D9通过选择性自噬中的Ca2 +信号传导调节TBK1激活。
可以通过异种吞噬选择性地消除入侵的微生物病原体。泛素介导的自噬受体被TANK结合激酶1(TBK1)磷酸化,并被募集到泛素化细菌以促进异种吞噬过程中自噬体的形成,但尚不清楚TBK1响应微生物感染而激活的分子机制。在这里,我们显示细菌感染通过Ca2 +结合蛋白TBC1域家族成员9(TBC1D9)增加了Ca2 +的水平以激活TBK1进行吞噬。从机械上讲,TBC1D9的泛素结合区(UBR)和Ca2 +结合基序介导其与泛素阳性细菌的结合,而TBC1D9敲除可抑制TBK1激活和随后的ULK1复合物募集。用Ca2 +螯合剂处理会损害异种吞噬过程中的TBC1D9-泛素相互作用和TBK1激活。TBC1D9还通过其UBR和Ca2 +结合基序募集到受损的线粒体,并且在线粒体吞噬过程中TBK1激活是必需的。这些结果表明,TBC1D9通过Ca2 +依赖性泛素识别控制异种和线粒体过程中的TBK1激活。

































 京公网安备 11010802027423号
京公网安备 11010802027423号