当前位置:
X-MOL 学术
›
Phytomedicine
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
β-Elemonic acid inhibits the growth of human Osteosarcoma through endoplasmic reticulum (ER) stress-mediated PERK/eIF2α/ATF4/CHOP activation and Wnt/β-catenin signal suppression.
Phytomedicine ( IF 6.7 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.phymed.2020.153183 Ang Zhao 1 , Zhanjie Zhang 1 , Yanfen Zhou 1 , Xin Li 1 , Xiaotian Li 2 , Bo Ma 1 , Qi Zhang 1
Phytomedicine ( IF 6.7 ) Pub Date : 2020-02-07 , DOI: 10.1016/j.phymed.2020.153183 Ang Zhao 1 , Zhanjie Zhang 1 , Yanfen Zhou 1 , Xin Li 1 , Xiaotian Li 2 , Bo Ma 1 , Qi Zhang 1
Affiliation
|
BACKGROUND
Osteosarcoma (OS) is a significant threat to the lives of children and young adults. Although neoadjuvant chemotherapy is the first choice of treatment for OS, it is limited by serious side-effects and cancer metastasis. β-Elemonic acid (β-EA), an active component extracted from Boswellia carterii Birdw., has been reported to exhibit potential anti-inflammatory and anticancer activities. However, the anti-tumor effects and underlying mechanisms on OS as well as pharmacokinetic characteristics of β-EA remain unknown.
PURPOSE
This study was aimed to investigating the anti-tumor effects of β-EA on human OS, the underlying mechanisms, and the pharmacokinetic and tissue distribution characteristics.
STUDY DESIGN AND METHODS
Cell viability and colony formation assays were performed to determine the effect of β-EA cell on cell proliferation. Apoptosis rates, mitochondrial membrane potential and cell cycle features were analyzed by flow cytometry. qRT-PCR, Western blot, immunofluorescence and immunohistochemical assays were conducted to evaluate the expression levels of genes or proteins related to the pathways affected by β-EA in vitro and in vivo. Cell migration and invasion were evaluated in wound healing and Transwell chamber assays. The effects and pharmacokinetic characteristics of β-EA in vivo were evaluated by analyzing tumor suppression, pharmacokinetics and tissue distribution.
RESULTS
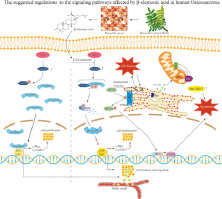
Explorations indicated that endoplasmic reticulum (ER) stress conditions provoked by β-EA activated the PERK/eIF2α/ATF4 branch of the unfolded protein reaction (UPR), stimulating C/EBP homologous protein (CHOP)-regulated apoptosis and inducing Ca2+ leakage leading to caspase-dependent apoptosis. Furthermore, β-EA induced G0/G1 cell cycle arrest and inhibited metastasis of HOS and 143B cells by attenuating Wnt/β-catenin signaling effects, which included decreased levels of p-Akt(Ser473), p-Gsk3β (Ser9), Wnt/β-catenin target genes (c-Myc and CyclinD1) along with a decline in nuclear β-catenin accumulation. The fast absorption, short elimination half-life, and linear pharmacokinetic characteristics of β-EA were also revealed. The distribution of β-EA was detected in the tumor and bone tissues.
CONCLUSIONS
Overall, both in vitro and in vivo investigations showed the potential of β-EA for the treatment of human OS. The pharmacokinetic profile and considerable distribution in the tumor and bone tissues warrant further preclinical or even clinical studies.
中文翻译:

β-榄香酸通过内质网(ER)介导的PERK /eIF2α/ ATF4 / CHOP激活和Wnt /β-catenin信号抑制来抑制人骨肉瘤的生长。
背景技术骨肉瘤(OS)对儿童和年轻人的生命构成重大威胁。尽管新辅助化疗是OS的首选治疗方法,但它受到严重副作用和癌症转移的限制。据报道,β-榄香酸(β-EA)是从乳香(Boswellia Carterii Birdw。)提取的活性成分,具有潜在的抗炎和抗癌活性。然而,对OS的抗肿瘤作用和潜在机制以及β-EA的药代动力学特征仍然未知。目的本研究旨在研究β-EA对人OS的抗肿瘤作用,潜在机制以及药代动力学和组织分布特征。研究设计和方法进行细胞生存力和集落形成分析,以确定β-EA细胞对细胞增殖的影响。通过流式细胞仪分析细胞凋亡率,线粒体膜电位和细胞周期特征。进行了qRT-PCR,Western印迹,免疫荧光和免疫组化分析,以评估与β-EA在体外和体内影响的途径相关的基因或蛋白质的表达水平。在伤口愈合和Transwell室分析中评估细胞迁移和侵袭。通过分析肿瘤抑制,药代动力学和组织分布,评估了β-EA在体内的作用和药代动力学特征。结果探索表明,β-EA引起的内质网(ER)应激条件激活了未折叠蛋白反应(UPR)的PERK /eIF2α/ ATF4分支,刺激C / EBP同源蛋白(CHOP)调节细胞凋亡并诱导Ca2 +泄漏,导致caspase依赖性细胞凋亡。此外,β-EA通过减弱Wnt /β-catenin信号转导作用(包括降低p-Akt(Ser473),p-Gsk3β(Ser9),Wnt的水平)诱导G0 / G1细胞周期停滞并抑制HOS和143B细胞的转移。 /β-catenin靶基因(c-Myc和CyclinD1)以及核β-catenin积累的下降。还揭示了β-EA的快速吸收,短消除半衰期和线性药代动力学特征。检测到β-EA在肿瘤和骨组织中的分布。结论总体而言,体外和体内研究均显示了β-EA在治疗人类OS中的潜力。
更新日期:2020-02-07
中文翻译:

β-榄香酸通过内质网(ER)介导的PERK /eIF2α/ ATF4 / CHOP激活和Wnt /β-catenin信号抑制来抑制人骨肉瘤的生长。
背景技术骨肉瘤(OS)对儿童和年轻人的生命构成重大威胁。尽管新辅助化疗是OS的首选治疗方法,但它受到严重副作用和癌症转移的限制。据报道,β-榄香酸(β-EA)是从乳香(Boswellia Carterii Birdw。)提取的活性成分,具有潜在的抗炎和抗癌活性。然而,对OS的抗肿瘤作用和潜在机制以及β-EA的药代动力学特征仍然未知。目的本研究旨在研究β-EA对人OS的抗肿瘤作用,潜在机制以及药代动力学和组织分布特征。研究设计和方法进行细胞生存力和集落形成分析,以确定β-EA细胞对细胞增殖的影响。通过流式细胞仪分析细胞凋亡率,线粒体膜电位和细胞周期特征。进行了qRT-PCR,Western印迹,免疫荧光和免疫组化分析,以评估与β-EA在体外和体内影响的途径相关的基因或蛋白质的表达水平。在伤口愈合和Transwell室分析中评估细胞迁移和侵袭。通过分析肿瘤抑制,药代动力学和组织分布,评估了β-EA在体内的作用和药代动力学特征。结果探索表明,β-EA引起的内质网(ER)应激条件激活了未折叠蛋白反应(UPR)的PERK /eIF2α/ ATF4分支,刺激C / EBP同源蛋白(CHOP)调节细胞凋亡并诱导Ca2 +泄漏,导致caspase依赖性细胞凋亡。此外,β-EA通过减弱Wnt /β-catenin信号转导作用(包括降低p-Akt(Ser473),p-Gsk3β(Ser9),Wnt的水平)诱导G0 / G1细胞周期停滞并抑制HOS和143B细胞的转移。 /β-catenin靶基因(c-Myc和CyclinD1)以及核β-catenin积累的下降。还揭示了β-EA的快速吸收,短消除半衰期和线性药代动力学特征。检测到β-EA在肿瘤和骨组织中的分布。结论总体而言,体外和体内研究均显示了β-EA在治疗人类OS中的潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号